- What’s it like to have ATTR-CM in Korea
- by won, jong-hyuk | translator Byun Kyung A | Dec 10, 2020 06:09am
And one day, he felt tingling sensation in his toes and examined the heart. That was the time he had to face his rare disease called hereditary transthyretin amyloid cardiomyopathy (ATTR-CM).
Typically categorized as either wild type or hereditary, ATTR-CM is an ultra rare disease that has extremely limited patient size and the prevalence rate in South Korea has not been estimated. To this date, about over 120 types of genetic mutation have been reported, and apparently some of them show qualities of endemic disease in specific regions.
But the biggest issue is that the disease is easily diagnosed as other disease.
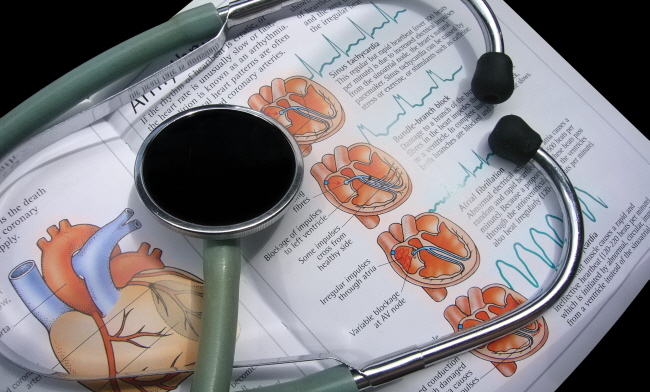
Some of the major symptoms of ATTR-CM include congestive heart failure, shortness of breath, edema, fatigue and more. And because of these symptoms, the rare disease is often misdiagnosed as unexpected restrictive cardiomyopathy, heart failure, irregular heartbeat and heart failure with preserved ejection fraction (HFpEF).
According to the researches done by academic societies so far, ATTR-CM is identified as a critical advanced rare disease resulting in restrictive cardiomyopathy caused by extracelluar deposition of transthyretin (TTR), normally involved in the transportation of the hormone thyroxine and retinol-binding protein, in the myocardium
Moreover, these patients’ prognosis could be worsened rapidly due to accumulated amyloid, and in fact, the survival period from the point of diagnosis is only about two to 3.5 years.
Considering the dire conditions, experts reiterate “An accurate early diagnosis is crucial for adequate disease management and improved treatment in patients with ATTR-CM.”
ATTR-CM treatment manages heart failure and irregular heartbeat, and uses treatments”
For the South Korean market, Vyndamax (tafamidis) is now indicated to treat adult patients with ATTR-CM based on its evidence of reducing the risk of heart-related death and cardiovascular issue-related hospitalization. The drug is the first and only treatment for the disease in South Korea.
The American Heart Association (AHA) recommends three treatment types for ATTR-CM—managing heart failure and irregular heartbeat, and using treatments.
Before the new indication approval, the only treatment was to manage the organ failure symptoms and to slow down the disease progress.
But ultimately, Vyndamax became a breakthrough treatment that patients can expect to reduce risk of cardiovascular-related death or hospitalization as the health authority cleared the drug.
The U.S. Food and Drug Administration (FDA) approved the treatment for patients with wild type or hereditary ATTR-CM, and more specifically the patients categorized as New York Heart Association (NYHA) Heart Failure Class I through III. The U.S. health authority reflected the recommendation of the drug contributing in delaying the disease progress when consuming Vyndamax at early stage.
Also, the Summary of Product Characteristics on tafamidis, published by the European Medicines Agency (EMA) mentions “To seek for more accurate clinical benefit in progress of ATTR-CM, tafamidis treatment should be initiated as soon as possible.”
Rate of misdiagnosis high in South Korea and delays actual treatment
The approval on Vyndamax was based on the results of a multicenter, placebo-controlled Phase III ATTR-ACT study with 441 patients with ATTR-CM.
The 441 patients were randomly assigned to administrating 80 mg or 20 mg of tafamidis or placebo. The primary endpoint evaluated the frequency of cardiovascular related death and hospitalization.
The secondary endpoint was the change in the Kansas City Cardiomyopathy Questionnaire Overall Summary (KCCQ-OS) score from 6-minute walk test taken and compared among the baseline time to 30-month point.

Moreover, the KCCQ-OS score and the 6-minute walk test at the 30-month point were improved significantly with the drug.
The president of the Korean Society of Heart Failure and a cardiology professor at Seoul National University Bundang Hospital, Dr. Choi Dong-ju emphasized, “ATTR-CM has high rate of misdiagnosis, which usually delays the diagnosis process. The ATTR-CM patients tend to only survive at least two to 3.5 years after the diagnosis, so an early diagnosis and aggressive treatment are important.”
Dr. Choi elaborated, “Although the studies and investigation on the disease is insufficient in South Korea, an effective treatment Vyndamax is already approved for prescription. If the patients can quickly get diagnosed, they would be able to manage their prognosis better. But because the drug is not listed for reimbursement, the financial burden on ATTR-CM patients in Korea is unfortunately very high. I hope the treatment access improve even by a bit as the disease has to be both urgently diagnosed and treated.”
Regardless of all efforts by the government and other organization, the only treatment option Vyndamax is still not listed for reimbursement in South Korea.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.
- [Reporter's View] Transparent info, the key to vaccine trust
- Reporter's view | Son, Hyung Min









