- Next Biomedical seeks IPO…knocks on global GI mkt
- by Hwang, Byung-woo | translator Kang, Shin-Kook | Aug 2, 2024 05:52am
The company has been collaborating with the global company Medtronic to expand into the endoscopic hemostatic agent market with its flagship product. Based on the revenue growth, the company plans to establish the drug as a standard-of-care in the global market.

Established in 2014, Next Biomedical is an innovative bio-solution company that develops therapeutic materials based on its polymer and drug delivery system technologies.
Its main products include an endoscopic hemostatic powder (Nexpowder), an endovascular embolization microsphere (Nexsphere), and an arthritis pain embolization treatment (Nexsphere-F).
Of these, the product that generates significant sales is the endoscopic hemostatic powder Nexpowder, which is a powder-type hemostatic treatment that can be used through endoscopes to hemorrhage bleeding sites and prevent rebleeding in the event of gastrointestinal bleeding.
In particular, Next Biomedical signed a global licensing agreement (excluding Korea, Japan, and Greater China) with Medtronic in 2020 to sell its products in 29 countries including the United States, Canada, and Europe.
The company's prominence in the digestive field can be attributed to CEO Lee's background as a professor of gastroenterology at Inha University Hospital.
Based on Lee’s clinical experience, the company was able to build a consortium of academic and university hospital collaborations and secure a network of key stakeholders in the GI field in Korea and abroad.
"The rise in endoscopic procedures has also led to the rise in GI bleeding amongst patients, rendering hemostasis and rebleeding prevention an important task for all surgeons including gastroenterologists,” said Lee. "After feeling the need while performing endoscopic procedures in the field, we developed a product, which is being exported to the US and Europe."
The company has succeeded in rapidly commercializing the finished product of the therapeutic material in Korea and abroad, which has shown results in terms of sales and exports.
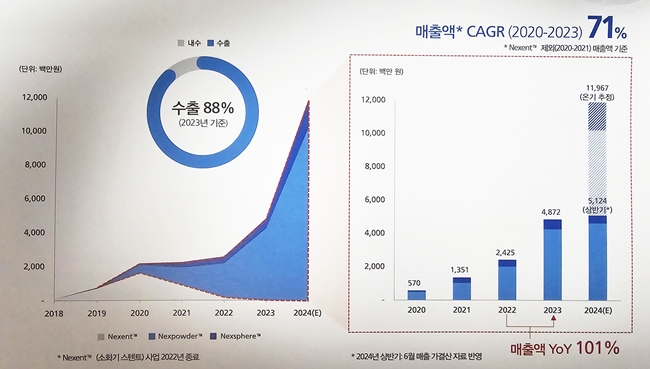
Last year, Next Biomedical posted sales of KRW 4.8 billion, which was nearly double the KRW 2.7 billion it had posted in 2022. In Q1 this year, sales were KRW 2.3 billion and are expected to exceed KRW 10 billion.
However, the company is still recording an operating loss due to an increase in SG&A expenses caused by the increase in R&D expenses. However, this is expected to turn into a profit after the IPO, supported by the growth in overseas sales in the U.S. and Europe, which accounted for 88% of sales last year.
"We estimate sales of about KRW 12 billion this year and consider the operating profit break-even point to be KRW 15 billion,” said Lee. "We don't have much cost burden because Dong-A ST is in charge of sales and marketing in Korea and Medtronic is in charge overseas, so we think we will able to record a surplus next year."
Will seek to enter the U.S. guidelines as a standard of care...is conducting post-marketing clinical trials
In particular, the company is conducting post-marketing clinical trials to enter the endoscopic hemostasis guidelines as a standard of care with its Nexpowder. It plans to build clinical evidence with 278 patients in 10 hospitals in the U.S., Canada, and Europe.

"We aim to be listed as a standard of care by utilizing the clinical evidence obtained from the clinical trial,” said Lee. "If the guidelines include the use of Nexpowder as a first-line treatment, the demand for the product is expected to increase significantly due to its differentiated competitiveness."
In addition, the company is focusing on occupying the market with Nexsphere-F, a fast-resorbable hydrophilic gelatin-based embolic microsphere for endovascular embolization. The drug reduces pain without side effects by embolizing abnormal blood vessels that cause arthritis pain with fast-acting microspheres that break down within a short period of time (2 to 6 hours).
Post-marketing clinical trials are already underway for the drug in Korea for a new health technology application, and post-marketing clinical trials are also soon to begin in Europe.
In addition, the company recently completed filing an Investigational Device Exemption application to the U.S. FDA to confirm the efficacy and safety of Nexsphere-F, with plans to receive approval by 2026.
"Based on our rapid product commercialization experience and abundant clinical evidence, we will take the initiative to open and preoccupy new markets by listing all our products as the global standard of care,” said Lee. “We will successfully enter the US market by completing the ongoing clinical trials and KOSDAQ listing."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









