- Potential blockbuster drug Kerendia…1yr since reimbursement
- by Whang, byung-woo | translator Alice Kang | Mar 4, 2025 05:57am
With its monthly sales recording KRW 1 billion in the last month of last year, the drug is rapidly building up its performance, and given its growth, it is likely to become a blockbuster treatment in Korea this year.
Kerendia, which was approved in Korea in May 2022, was approved for insurance reimbursement benefits on February 1 of last year, about a year and a half after its approval in Korea.
The standards for Kerendia’s reimbursement are as follows: Adult patients with type 2 diabetes who have been taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) for more than four weeks, but have ▲a urinary albumin-to-creatinine ratio (uACR) over 300 mg/g or a urine dipstick test result of 1+ or higher, and ▲an estimated glomerular filtration rate (If eGFR is between 25 and 75, the drug may be administered together with ACE inhibitors and ARBs).
Kerendia gained the spotlight as diabetes accounts for the highest proportion (38.6%) among the causes of end-stage renal disease.
Until now, drugs to control blood pressure and reduce the burden on the kidneys were used with GLP-1 and SGLT-2 inhibitors to control blood sugar, but there was an unmet need.
In particular, as there had been no drug that could directly suppress chronic inflammation or fibrosis of the kidneys, Kerendia, which has a new mechanism of action that can directly target these, is expected to play a greater role.
“So far, GLP-1 receptor agonists, SGLT-2 inhibitors, and RAS inhibitors, which are blood pressure medications, have been used to treat patients with diabetes and kidney disease, but the risk of chronic kidney disease persisted in the patients,” said Yong-Ho Lee, Professor of endocrinology at Severance Hospital and Director of General Affairs of the Korean Diabetes Association. “As Kerendia has proven its efficacy in various clinical trials, it will become an important treatment option for patients with chronic kidney disease,” he said.
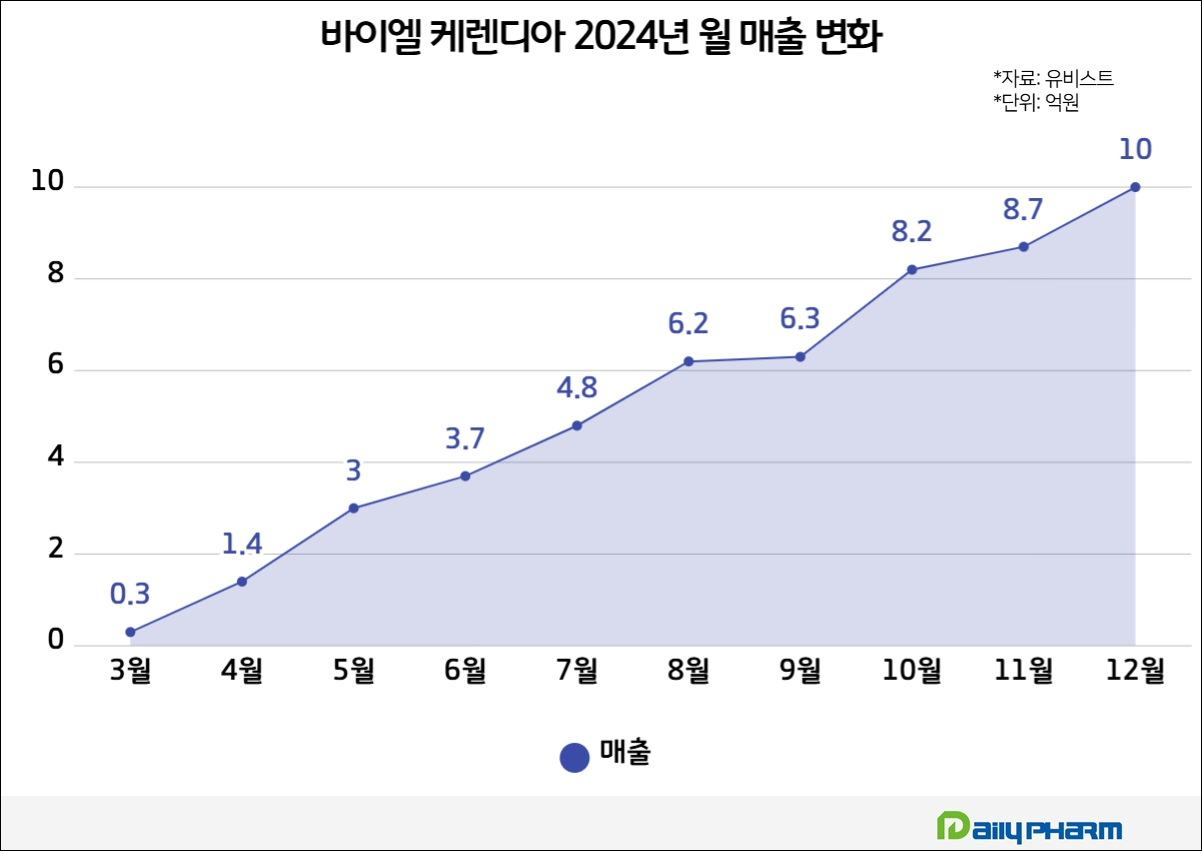
In fact, Kerendia‘s sales have been growing since its reimbursement in Korea.
After being granted reimbursement last year, Kerendia’s monthly sales started at KRW 30 million in March and continued to rise, reaching KRW 100 million in April. In particular, the company recorded monthly sales of KRW 1 billion in December, the last month of last year, and is expected to continue to increase its influence this year.
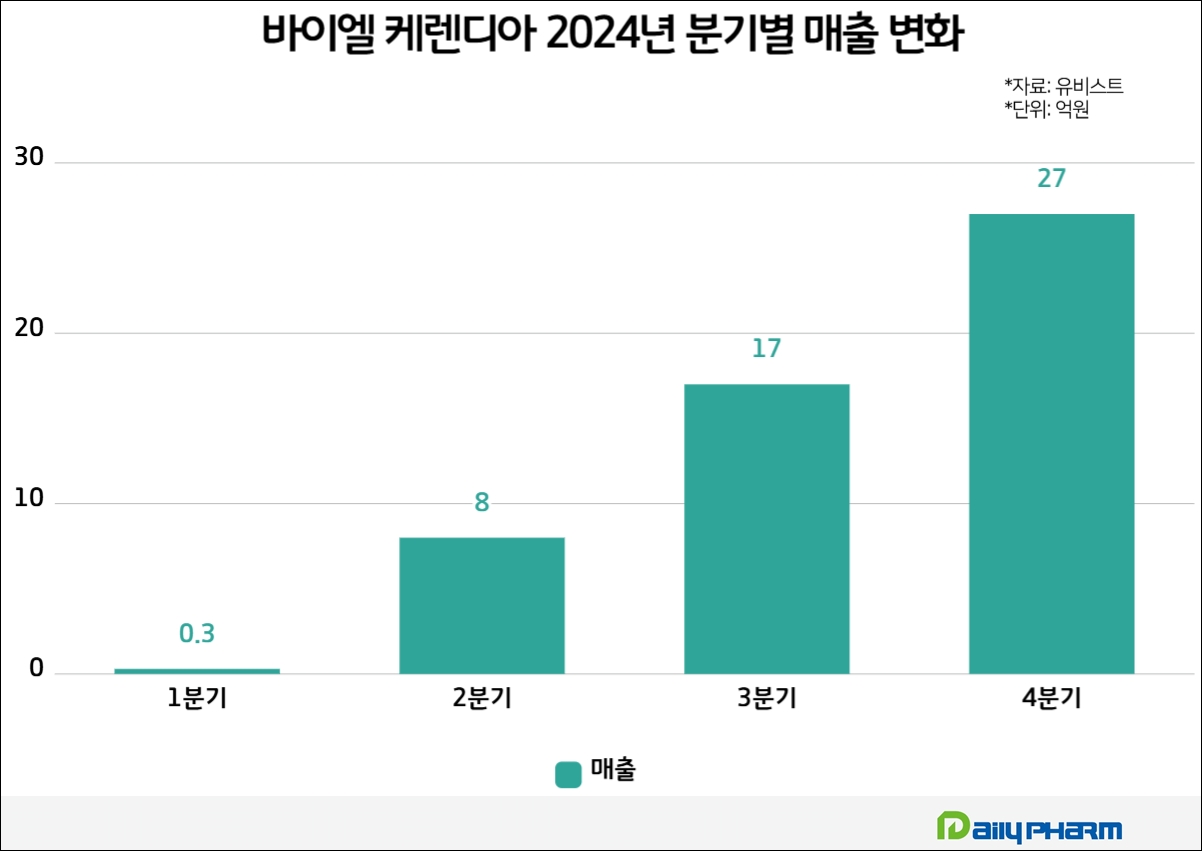
Looking at the quarterly figures, the company showed twofold growth each quarter, posting KRW 800 million in the second quarter, KRW 1.7 billion in the third quarter, and KRW 2.7 billion in the fourth quarter.
Taking this into account, sales are expected to easily exceed the KRW 10 billion mark which is the threshold for a blockbuster drug in Korea, within this year.
The expansion of the prescription of Kerendia seems to have been influenced by its characteristics as a treatment for chronic kidney disease patients with Type 2 diabetes.
According to UBIST, prescriptions increased significantly in the early days after the reimbursement was applied, mainly in the field of nephrology, but the number of prescriptions in the field of endocrinology also increased from September, and in December, when monthly sales reached KRW 1 billion, the number of prescriptions in the field of nephrology and endocrinology did not differ significantly.
In this situation, the drug’s influence in the market is expected to continue to grow as Bayer Korea announced plans to expand Kerendia’s indications.
On November 20, 2019, the Ministry of Food and Drug Safety approved a randomized clinical trial to determine the efficacy and safety of finerenone on the morbidity and mortality of heart failure patients with a left ventricular ejection fraction of 40% or more who were hospitalized due to an acute non-reversible episode of heart failure.
It can be regarded as a domestic version of the Phase III FINEARTS-HF trial, which evaluated Kerendia in patients with heart failure with a left ventricular ejection fraction of 40% or more, which was announced at the European Society of Cardiology's annual conference (ESC 2024) in September last year.
As Kerendia has already been shown to prevent heart failure-related secondary events in patients with heart failure with reduced ejection fraction (HFmrEF) and heart failure with preserved ejection fraction (HFpEF), which have ejection fraction 40% or higher, the indication expansion in Korea is not expected to be difficult if there are no major variables.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









