- ADC mkt leader Daiichi-Sankyo's R&D drive
- by Whang, byung-woo | translator Hong, Ji Yeon | Mar 27, 2025 05:54am
Daiichi-Sankyo plans to focus on expanding and strengthening access through the DXd-ADC platform, which is a basis for the company's chief pipeline.

Recently, Enhertu received an expanded indication from the U.S. Food and Drug Administration (FDA) for the treatment of patients with 'HER2-low metastatic breast cancer who have been treated with one or more endocrine therapy sessions' and expanded its market impact.
The DXd-ADC platform has been the basis of this achievement. Daiichi-Sankyo, launched after a merger between Daiichi and Sankyo, strengthened ADC technology based on a synergy between Sankyo's monoclonal antibody (mAb) technology and Daiichi's anticancer agent payload and linker technology.
Previously, conventional ADCs have been limited in therapeutic effects due to ▲Lack of payload diversity ▲Heterogeneity in drug conjugation sites ▲Linker instability ▲Restrictions on the number of drugs that can be conjugated.
The DXd-ADC platform gained attention because it offered improved anticancer effects and overcame existing ADC limitations.
The DXd-ADC platform has been used to expand the pipeline in addition to Enhertu based on seven technological strengths, including ▲High-potency payloads ▲High drug-to-antibody conjugation ratios ▲Uniform binding between the drug and antibody.
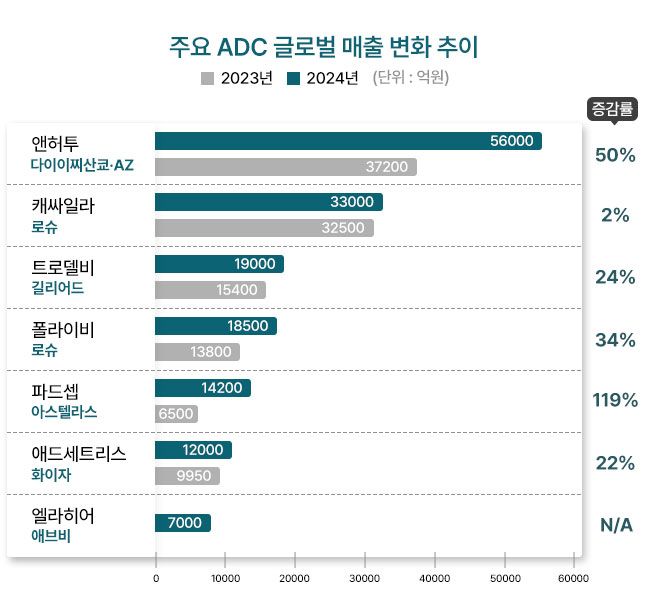
Enhertu, jointly-developed by Daiichi-Sankyo and AstraZeneca, secured the most indications and ranked the top among new ADC drugs.
Enhertu recorded the highest sales in the U.S. market last year, with U.S. sales reaching 282.4 billion yen, a 36.0% increase year-over-year, and the U.S. market is expected to account for 49.0% of Enhertu's total sales in 2024.
In South Korea, Daiichi-Sankyo Korea recorded sales of KRW 274 billion in 2023, the highest among Japanese pharmaceutical companies operating in South Korea. Given Enhertu's growth, its overall sales are projected to exceed KRW 300 billion in 2024.
Daiichi-Sankyo is preparing for a new drug that is Enhertu's follow-up…focusing on expanding the pipeline
Daiichi-Sankyo has been employing a '5 DXd-ADC & Next Wave' strategy, focusing its R&D capacity on five ADCs in the oncology sector and the 'Next Wave' pipeline, including products for rare diseases and vaccines.
Daiichi-Sankyo's chief pipeline includes ▲TROP2-directed Dato-DXd (datopotamab deruxtecan) ▲HER3-directed HER3-DXd (patritumab deruxtecan) ▲B7-H3-directed I-DXd (ifinatamab deruxtecan) ▲CDH6-directed R-DXd (raludotatug deruxtecan).
A Trop-2-directed antibody drug, Datroway, is Enhertu's follow-up drug, and it was approved by the Food and Drug Administration (FDA) in January as a breast cancer medication. Datroway is soon to be launched.
Datroway can be used as a treatment for hormone receptor (HR)-positive and human epidermal growth factor 2 (HER)-negative breast cancer.
Furthermore, patritumab deruxtecan is a drug candidate that is expected to receive approval this year. Clinical trials of patritumab and ifinatamab are being conducted involving patients with lung cancer and ovarian cancer, respectively.
In addition, Daiichi-Sankyo is focusing on securing next-generation growth drivers by developing innovative therapies in the specialty drug and vaccine sectors, leveraging its proprietary modality technology.

The 'Expand' strategy aims to establish a DXd-ADC treatment approach for breast and lung cancers and subsequently expand its indications to earlier treatment stages and a broader range of cancer types. Meanwhile, the 'Extend' strategy encompasses the development of next-generation ADCs and new modalities that maximize platform effects through combination therapies and formulation changes. Thus ultimately providing additional treatment options beyond DXd-ADC therapy.
Jeong-tae Kim, CEO of Daiichi-Sankyo Korea, stated, "As of 2024, over 40 of our global clinical studies have been conducted in South Korea, and two Korean medical institutions have been selected among 15 Phase 1 clinical sites across eight countries, contributing from the earliest stages of clinical research."
"We plan to launch four ADCs and targeted cancer agents and have substantially prepared and put efforts into leaping as a leader in the oncology sector. For instance, providing treatment options to more patients," Kim added, "In addition to distributing pharmaceuticals, we will provide pragmatic hope by leading innovations in collaboration with Korean medical communities and acting as a bridge with the headquarters."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









