- AbbVie Korea seeks to grow to ₩KRW 300B with biodrugs
- by Whang, byung-woo | translator Alice Kang | Mar 31, 2025 05:59am

The company is working to improve its capabilities by balancing the 3 key factors - sales growth, R&D, and social contribution.
AbbVie Korea was established in 2013 as the Korean affiliate of AbbVie, headquartered in North Chicago, Illinois, USA.
Its major business units include ▲the Immunology Business Unit (rheumatoid diseases, psoriasis, atopic dermatitis, inflammatory bowel diseases, etc.), ▲the Specialty Business Unit (hepatitis C, chronic migraine, etc.), and ▲the Oncology Business Unit, each of which has a solid portfolio.
AbbVie eliminates the risk of Humira... Skyrizi and Rinvoq shows shared growth
AbbVie’s representative product has long been Humira, a blockbuster immune disease treatment. It was a highly symbolic product as it has been the global No. 1 specialty drug in the market for the past 10 years.
However, in recent years, Humira has also been AbbVie’s biggest concern as well. With the looming entry of Humira biosimilars upon the expiration of its patent, there were doubts about whether the company would be able to address Humira’s expected sales gap.
In fact, when competition with biosimilar products intensified upon the expiry of Humira's North American patent in 2023, there were concerns about the company’s sales recovery, as sales fell by USD 5.4 billion (about KRW 7 trillion) year-on-year.
To conclude, the company has eliminated Humira’s sales risk.
Although sales of Humira were inevitably reduced, the loss was quickly made up due to the growth of the company’s follow-up drugs, next-generation immune disease treatments ‘Rinvoq’ (Upadacitinib) and ‘Skyrizi’ (Risankizumab).
According to the 2024 Global Pharmaceutical Sales Rankings, Skzrizi recorded sales of USD 11.72 billion (KRW17.2237 trillion), a 50.9% increase from the previous year, ranking seventh among all products. This year, Skyrizi’s sales are expected to reach USD 13.72 billion (KRW 20.162.9 trillion)
Rinvoq’s sales target is also up to USD 2 billion (about KRW 2.9 trillion) for this year and 2026, the success of these two follow-up drugs is demonstrating AbbVie’s strong foothold in the field of immune diseases.
Thanks to the growth of Skyrizi and Rinvoq, AbbVie recorded USD 54.5 billion in sales in 2024, ranking second in the global pharmaceutical industry in terms of sales.
The stock price also reflected this expectation of sales, recording a high growth of over 34% as of March 1, 2025, compared to two years ago, March 1, 2023.
In response to this, Robert Michael, CEO of AbbVie, said, “We expect net profit to exceed the previous high, in just 2 years after the expiration of the Humira patent in the United States.”
Unlike how other pharmaceutical companies usually take 9-11 years to recover sales after the expiration of their blockbuster patents, AbbVie’s sales are expected to recover in just two years, successfully turning the crisis of patent expiration into an opportunity.
The company’s new drugs still lack influence in Korea... The cross-administration reimbursement approval for atopic dermatitis drugs expected to be beneficial
Even in the domestic market, the sales fluctuations of Humira have decreased, while sales of Rinvoq and Skyrizi grew rapidly.
According to the market research institution IQVIA, Humira recorded sales of KRW 104 billion in 2020 and surpassed the KRW 100 billion mark, but saw its sales drop to KRW 91.2 billion in 2021.
This is the combined result of the drug price cuts and market competition following the launch of biosimilars in Korea in June 2021. Since then, Humira has recorded sales of KRW 85.8 billion in 2022 and KRW 86.6 billion in 2023 and entered a stable sales period.
In this situation, Skyrizi recorded sales of KRW 27.9 billion in 2023, an increase of KRW 11.4 billion from KRW 16.5 billion in 2022, while Rinvoq also recorded sales of KRW 20.7 billion, an increase of KRW 9.2 billion from KRW 1.1 billion in 2022.
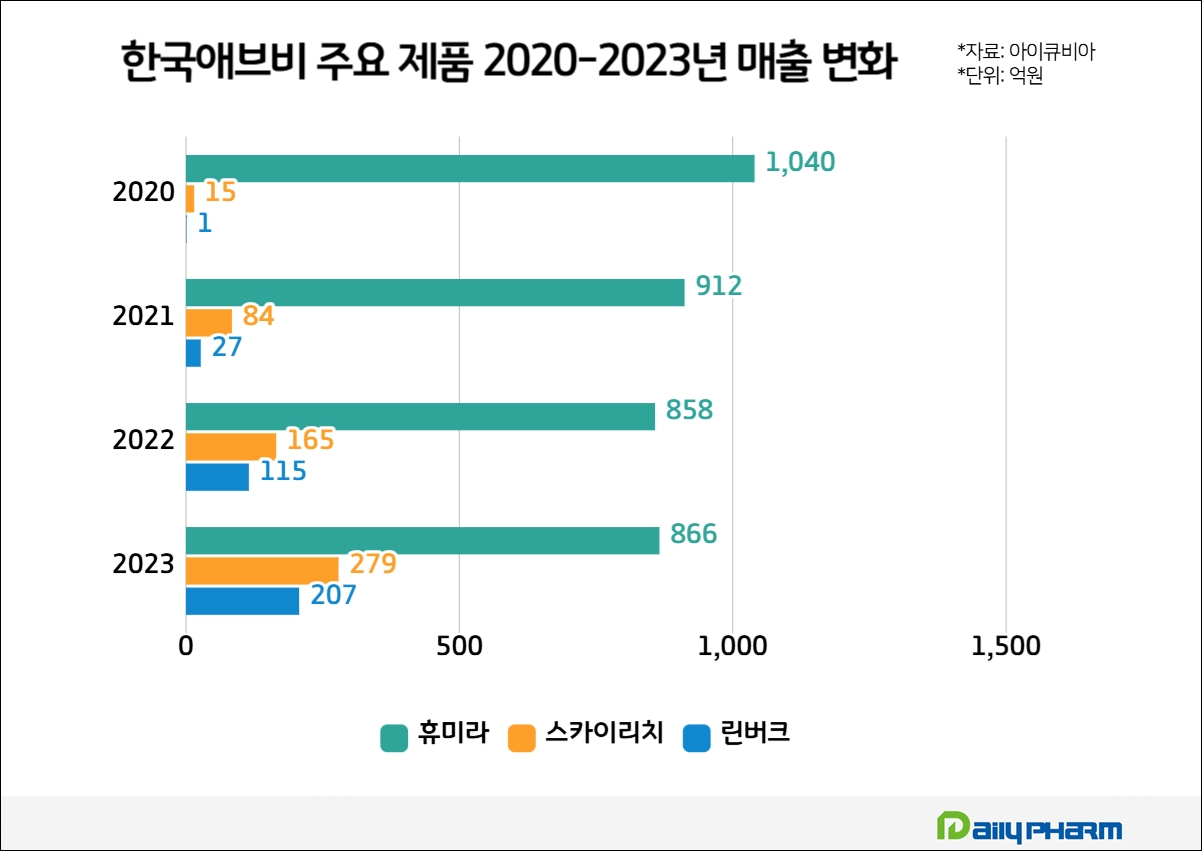
Although the overall scale of the drugs’ sales is still small compared to Humira's, when considering that the sales of ‘Rinvoq+Skyrizi’ have reached half the level of Humira's, rising from KRW 28 billion in 2022 to KRW 48.6 billion in 2023, there is a good chance that it will overtake Humira's sales within a few years.
In particular, there are high expectations on Rinvoq’s growth , as reimbursement for cross-administration between biological drugs and JAK inhibitors is now granted for severe atopic dermatitis in Korea.
The reimbursement approval for cross-administration of the drugs is expected to change the monopoly made by the biological drugs that entered the market the earliest. Many predict that Rinvoq will be the biggest beneficiary, and the drug is expected to continue its strong growth.
However, the company is concerned that the overall sales growth of AbbVie Korea is not as large as expected.
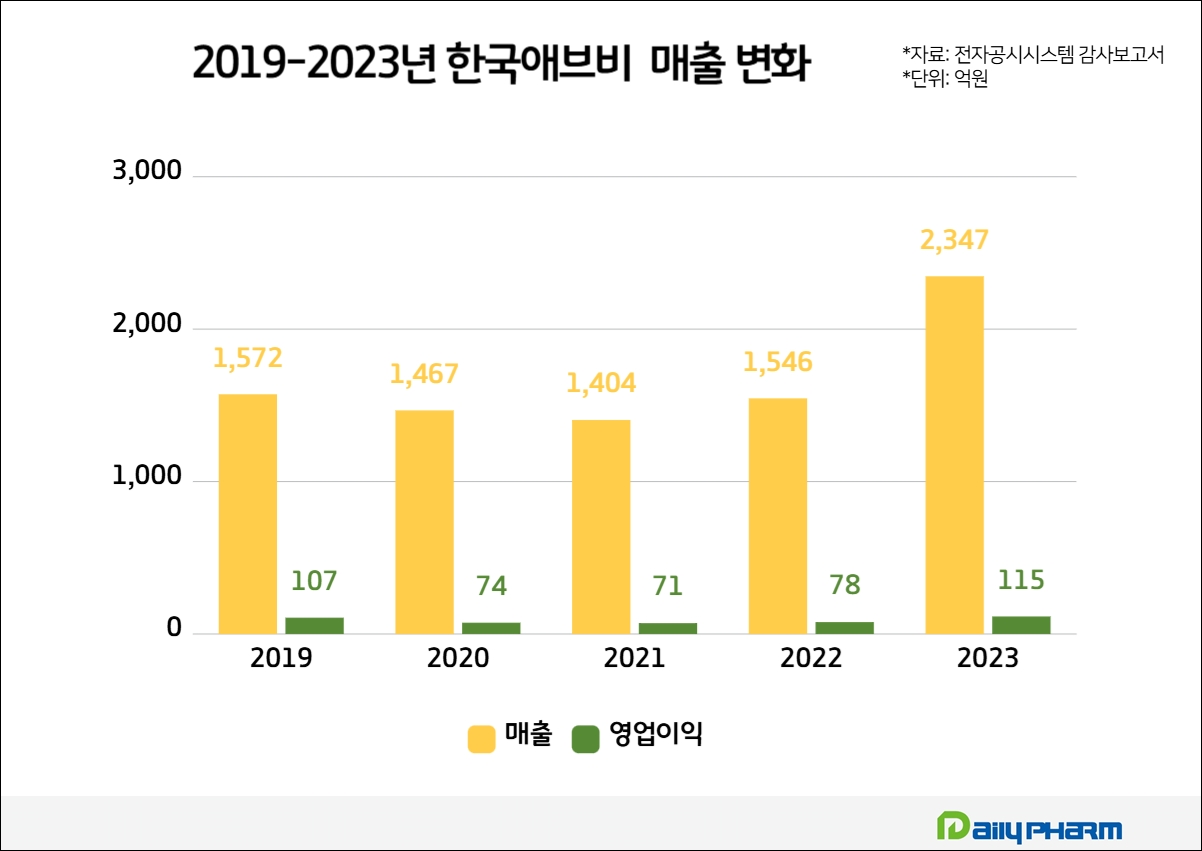
According to the audit report disclosed on the Data Analysis, Retrieval, and Transfer System, DART, AbbVie Korea's posted KRW 234.7 billion in sales in 2023. Its operating profit was KRW 11.5 billion. This is an increase of about KRW 80 billion compared to KRW 154.6 billion in 2022, but this is no major change, considering its absorption merger with Allergan Korea last year.
As such, the audit report released in early April is expected to be an indicator of whether the sales of Humira, which entered a stable period in 2024 and the growth of new drugs will be able to create synergies.
AbbVie expands its portfolio... Strengthening global competitiveness
Nevertheless, the reason why the industry has high expectations for the future of AbbVie is because it is expanding its pipeline along with its strong position in the field of immunology.
Following the launch of Venclexta, a treatment for acute myeloid leukemia and chronic lymphocytic leukemia, the company is working to get Epkinly, a treatment for relapsed or refractory diffuse large B-cell lymphoma (DLBCL) it received approval last year, reimbursed in Korea.
In addition, Elahere (mirvetuximab soravtansine), for which the company recently announced the results of a global Phase III clinical study, is also attracting attention as the first-in-class drug.
Ovarian cancer is mostly detected in the late stages, and platinum-based chemotherapy is considered as its first-line treatment. However, there is no other available treatment option if resistance develops during the first treatment, so Elahere is expected to play an important role in the treatment of platinum-resistant ovarian cancer in the future.
In addition, the company has signed a license agreement with the Danish company Gubra to develop a new drug for the treatment of obesity and secured GUB014295, a long-acting amylin analogue, which is regarded as the next generation of obesity treatment.

Meanwhile, since its foundation, AbbVie Korea has been steadily practicing sharing and volunteer activities for patients with rare and intractable diseases and the underprivileged, striving to fulfill its corporate social responsibility.
The company’s representative social contribution program is the “Week of Possibilities,” which has been participated in by employees around the world since its founding in 2013.
Specifically, the company has been carrying out various activities, including pop art portraits that brightly depict patients with rare and incurable diseases whose self-esteem has been lowered due to a long period of illness, a mosaic of air-purifying plants (scandia moss) for climate-vulnerable groups, and tree planting to reduce global warming and create healthy forests together.
In addition, A-Walk, which was launched in 2016, is a walking campaign by employees to help patients and has been praised for contributing to the improvement of employees' health and strengthening teamwork through innovative ideas.
Under the program, when employees achieve their target number of steps, matching donations are made for patients. Last year, the event was expanded to include employees from 8 Asian countries who participated in A+Walk and donated to patient organizations in each country.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









