- What are the remaining issues for Leclaza?
- by Moon, sung-ho | translator Hong, Ji Yeon | Apr 1, 2025 05:53am
As results indicate improvements in progression-free survival (PFS) as well as overall survival (OS) compared to Tagrisso (osimertinib, AstraZeneca), Janssen has initiated patient programs at major hospitals.
According to industry sources on March 31, the MARIPOSA Phase 3 study results presented at the European Lung Cancer Congress (ELCC 2025) in Paris have established combination therapy as a global standard.
The study demonstrated that, compared to Tagrisso monotherapy, the combination therapy extended OS by more than one year, offering outstanding clinical benefits. Attention is shifting to how quickly this combination therapy can be adopted in clinical settings.
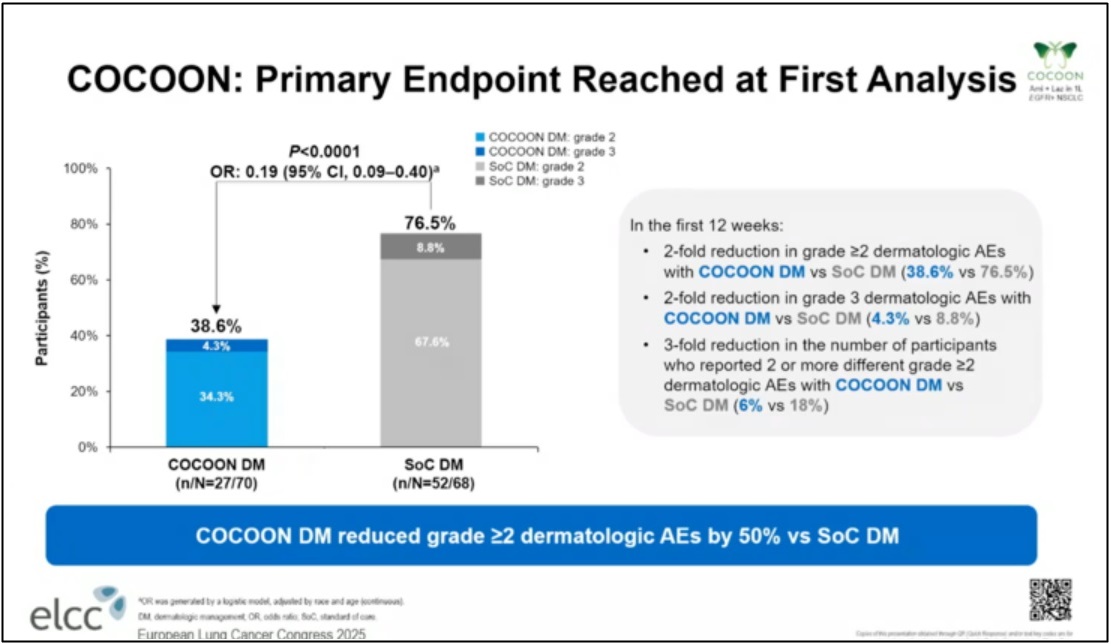
In addition, the interim analysis of the 'Cocoon' clinical trial, also presented at ELCC 2025, deserves particular attention, as it may provide solutions to typical side effects associated with combination therapies, such as skin rash and periungual inflammation.
The Cocoon study was designed for 200 patients with treatment-naïve metastatic non–small cell lung cancer (NSCLC) with EGFR mutations. In the interim analysis, 138 patients were included, with participants divided into a standard-of-care (SOC) group and a prophylactic management group, both receiving the combination therapy.
The study's prophylactic group designated to receive 'Cocoon therapy' received a comprehensive prevention strategy including ▲Oral administration of doxycycline or minocycline at 100 mg for weeks 1 to 12 ▲Application of a clindamycin lotion to the scalp from weeks 13 to 52 ▲Chlorhexidine cleansing of the nails ▲Use of ceramide-based moisturizers on the body and face. The SOC (standard-of-care) group was managed reactively with ltreatments, such as topical steroids or antibiotics, as needed based on local clinical practices.
The primary endpoint was the incidence of Grade ≥ 2 skin adverse events within 12 weeks after treatment initiation. At the interim analysis, over 70% of all patients had completed the 12‑week assessment. In the prophylactic 'Cocoon therapy' group, the incidence of Grade ≥ 2 skin adverse events was 38.6%, more than half that observed in the SOC group (76.5%).
As a result, only 21% of patients in the prophylactic group required a dosage reduction of the combination therapy due to side effects, compared to SOC group (31%). Similarly, treatment discontinuation due to adverse events occurred in only 11% of patients in the prophylactic group versus 19% in the SOC group.
Notably, for skin-related adverse events, only 7% of patients in the prophylactic group needed to reduce the dosage of Leclaza or Rybrevant compared to 19% in the SOC group, with discontinuation rates of 1% versus 4%, respectively.
Patients who discontinued treatment due to adverse reactions were 11% versus 19%, which was nearly half.
Overall, the study results demonstrate that the Cocoon therapy offers an effective solution for managing the skin-related side effects that have long been a significant concern with the combination therapy. Analysis suggests that it could potentially improve treatment continuity.
Professor Byoung Chul Cho, Director of the Lung Cancer Center at Yonsei Cancer Hospital, said, "With the overall survival results for the combination therapy presented at ELCC 2025, there is a common view among experts that this therapy could be selected as the preferred therapy in the NCCN guidelines given its significant clinical benefit. Given these impressive clinical outcomes, the drug must manage the side effects effectively."
"The Cocoon study has provided a viable approach for managing skin rashes," Professor Cho added, "In clinical practice when a combination therapy improves survival by one year over the current standard of care, we cannot dismiss it simply because it requires more intensive side effect management. With the drug's proven efficacy, further discussion is needed on adopting this more effective combination therapy."
As for clinical practices in South Korea, the biggest hurdle for Leclaza-Rybrevant combination therapy is related to its cost.
Leclaza monotherapy has been reimbursed with the National Health Insurance coverage since last year. However, Lecalza in combination with other drugs is still non-reimbursed.
Professor Sun Min Lim (Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Hospital), said, "Although the therapy is legally available since it has received domestic approval, the high cost makes it difficult to consider," adding, "Based on clinical data, treatment isn't limited to just one year. Patients may need to be treated for up to 50 months, a significant burden from the patient's perspective."
Meanwhile, Janssen has initiated patient programs at major hospitals capable of utilizing the combination therapy, starting mid-March.
Janssen has confirmed that the company will cover 72% of the drug price of Leclaza–Rybrevant combination therapy for the first 12 vials and 20% for each vial from the 13th onward.
For Leclaza, the drug price is refunded to patients according to a risk-sharing agreement negotiated by Yuhan with the regulatory authorities last year. As a result, only Rybrevant's cost is supported through patient assistance programs.
Professor Cho said, "Although there are price hurdles, compared to other options, the Leclaza-Rybrevant combination therapy preserves a chemotherapy option for future resistance," adding, "In comparison to treatments that are advanced from first-line therapy, it offers the advantage of providing viable second- and third-line treatment options for patients."
Doctors voice that policy reforms are needed to address the increasing prevalence of combination therapies.
In fact, over the past five years, 54 combination therapy for anticancer drugs have been approved, of which 26 involve combinations between two new drugs, similar to the Leclaza-Rybrevant combination therapy.
The healthcare authorities have explained that reimbursement discussion is underway, considering the approvals of various anticancer combination therapies, the overall sequencing of treatment lines, and the number of administrations.
Kim Gook-hee, Head of the Pharmaceutical Benefits Department at HIRA, said, "Anticancer drugs are clearly defined from the approval documentation regarding regimen and treatment sequence, and reimbursement criteria are set accordingly," adding, "However, with the recent surge in combination therapies, there are concerns about whether this approach can be maintained and whether all such combinations should be covered under reimbursement."
Kim added, "For anticancer drugs, discussions are already underway in the Cancer Drug Review Committee considering the regimen, treatment line, and overall survival period," adding, " Although side effects have decreased, we must also comprehensively consider the toxicity issues that can arise when anticancer drugs are combined."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









