- Policy
- High conc Ultomiris Inj 100mg/mL also approved in Korea
- by Kim, Jung-Ju Jan 2, 2023 06:04am
- Handok received marketing authorization for the orphan drug ‘Ultromiris 100mg/mL(ravulizumab)’ that is used to treat Paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uraemic syndrome (aHUS) and made a step towards supplying the drug in Korea. On the 28th, the Ministry of Food and Drug Safety announced that it had grante
- Policy
- Australia, excluding drug price reference countries
- by Lee, Tak-Sun Jan 1, 2023 10:40pm
- Australia's addition to the drug price reference country, which faced opposition from the pharmaceutical industry, failed. The HIRA initially decided to take a step back from adding Australia and Canada to the drug price reference country and add only Canada to the reference country. The HIRA released the "Detailed Evaluation Standards for Dr
- Policy
- New formulations of narcotics will be as strictly reviewed
- by Lee, Jeong-Hwan Dec 30, 2022 06:33am
- Regulations on narcotics for medical use, such as narcotic appetite suppressants and propofol, which is used for general anesthesia, that the government is restricting new approvals for, are expected to become stricter than before. Until now, even narcotic medications for which new approvals are restricted by the government were allowed
- Policy
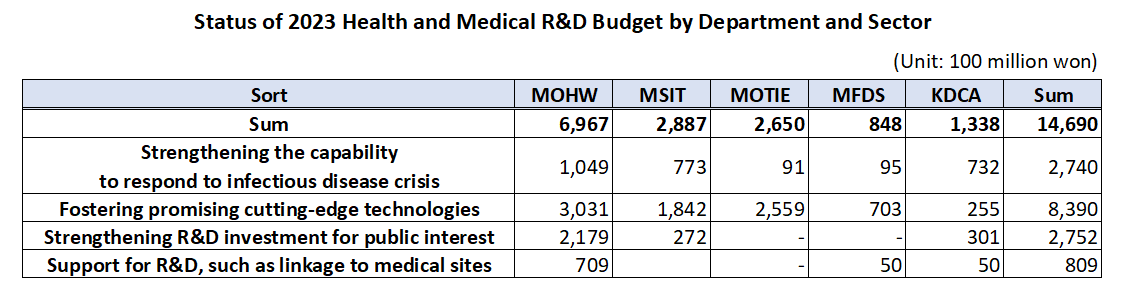
- Gov allocates KRW1.47 trillion budget for healthcare R&D
- by Kim, Jung-Ju Dec 30, 2022 06:33am
- The government’s budget for next year’s healthcare R&D including new drugs, medical devices, and digital transformation to AI-based biohealth is estimated to be around KRW 1.47 trillion. This is the total amount of budget that will be supported by the Ministry of Health and Welfare, Ministry of Science and ICT, Ministry of Trade, Industry, and
- Policy
- A rapid change in the population
- by Kang, Shin-Kook Dec 29, 2022 06:03am
- Policy Tasks Determined at the Second Vice-Minister Meeting of the Ministry Related to Population Future Strategy. The government will come up with all-around measures due to population changes caused by the world's highest rate of low birth rate and aging society. This included institutionalization of non-face-to-face treatment and visiting med
- Policy
- Takeda CMV infection treatment Livtencity has been approved
- by Kim, Jung-Ju Dec 29, 2022 06:03am
- After transplantation of Takeda Pharmaceutical Korea, Cytomegalovirus infection treatment Livtencity obtained domestic item permission and passed the first gateway to supply. The Ministry of Food and Drug Safety (Director Oh Yoo-kyung) announced on the 27th that it has approved Livtencity of Takeda, a rare drug. Cytomegalovirus (CMV) is as
- Policy
- Abbott’s Lipidil NT 145mg listed with reimbursement
- by Lee, Tak-Sun Dec 27, 2022 06:10am
- The original developer of the hyperlipidemia treatment fenofibrate will be releasing a 145mg product that can be taken on an empty stomach. This is the second drug to be released, following Yuhan Corp. As such, the two products are expected to compete fiercely in the market next year. According to industry sources on the 26th, Abbott
- Policy
- Darzalex succeeded in renewing his contract with RSA
- by Kim, Jung-Ju Dec 26, 2022 06:07am
- Darzalex, a treatment for multiple myeloma by Janssen Korea, has succeeded in negotiating a contract renewal with the NHIS. As a condition for renewal of the contract, the drug price was reduced by 2% by content, and RSA plans such as refund rate and cap were also set. According to the industry, the Ministry of Health and Welfare will push fo
- Policy
- Dong-A ST’s Forxiga prodrug Dapapro 5mg listed for reimb
- by Lee, Tak-Sun Dec 26, 2022 06:07am
- Following the reimbursement listing of the 10mg formulation of Dapapro Tab, the first follow-on drug of the antidiabetic SGLT-2 inhibitor Forxiga that had been developed by Dong-A ST, the 5mg lower-strength formulation of the drug is also soon to be listed with reimbursement. When listed, Dapapro Tab 5mg will be the only dapagliflozin dru
- Policy
- Zavicefta has been approved for domestic use
- by Lee, Hye-Kyung Dec 26, 2022 06:06am
- .The Ministry of Food and Drug Safety (Director Oh Yu-kyung) announced on the 22nd that it has approved Korea Pfizer Pharmaceutical's "Zavicefta 2g/0.5g (Ceftazidime/Avibactam)," a new antibiotic drug. This drug is a combination of Cefalosporin-based antibiotic Ceftazidime and avibactam, a newly developed beta-lactam inhibitor. Permitte