- Policy
- Exclude Australia from the drug price reference country?
- by Lee, Tak-Sun Dec 26, 2022 06:06am
- Insurance authorities, which are seeking to expand drug reference countries from seven to nine from next year, are expected to exclude Australia from strong opposition from the pharmaceutical industry. It is said that the government has turned to a careful review after a meeting with the Vice Minister of Health and Welfare and the pharmaceuti
- Policy
- Revlimid's benefit for multiple myeloma maintenance therapy
- by Lee, Tak-Sun Dec 23, 2022 06:05am
- Revlimid, a treatment for multiple myeloma, is eligible for maintenance therapy for hematopoietic stem cell transplantation patients. The effective date is January 1 next year. The HIRA has launched an opinion survey on the revision of the anti-cancer drug standard. According to the revision, benefits will be newly established for maintena
- Policy
- Lucentis biosimilar price set 63% lower than original
- by Lee, Tak-Sun Dec 23, 2022 06:05am
- Biosimilars of the macular degeneration treatment Lucentis (ranibizumab), which are set to be listed for reimbursement in Korea next month, are expected to be launched at a price much lower than their original. As a result, the burden borne by these patients is expected to be reduced significantly. According to industry sources on the 21s
- Policy
- New reimbursement standards set for Kymriah·Zolgensma
- by Kim, Jung-Ju Dec 22, 2022 05:52am
- New reimbursement standards have been set for high-priced pharmaceuticals like Kymriah and Zolgensma, the so-called ‘one-shot treatments.’ Also, reimbursement standards will be newly established for the second CGRP-targeted therapy for migraine, Ajovy (fremanezumab), and the new tuberculosis drug Dovprela 200mg (pretomanid) that had bee
- Policy
- Lucentis biosimilar enters KRW 35B market in Korea
- by Lee, Tak-Sun Dec 21, 2022 06:05am
- Competition in the macular degeneration treatments market is expected to intensify with the imminent entry of the first biosimilar of ‘Lucentis’ in the Korean market. Sales of Lucentis alone had been nearly KRW 35 billion in Korea last year. Lucentis biosimilars from Chong Kun Dang and Samsung Bioepis were preannounced to be listed fo
- Policy
- Shingrix will be released as a national lot on the 16th
- by Lee, Tak-Sun Dec 21, 2022 06:05am
- Shingrix (GSK), a shingles virus vaccine, received a national lot release on the 16th and began full-scale vaccination. It was also stocked in general hospitals, and vaccinations began this week. On the 16th, the Ministry of Food and Drug Safety released four production numbers (six in total, 0.5ml in packaging) with an expiration date of Oct
- Policy
- Pfizer & Novartis generic drugs disappear from the market
- by Lee, Tak-Sun Dec 20, 2022 06:06am
- All generic drugs released by Novartis and Pfizer Korean branches in the domestic market have disappeared. Although it was successfully released, it is interpreted that it left the market after losing a lot of competition with domestic pharmaceutical companies. According to industries on the 19th, Novartis' Pneumast 10mg and Pneumast 3mg w
- Policy
- President Yoon said, block medical shopping
- by Kang, Shin-Kook Dec 20, 2022 06:05am
- As concerns arose that it might lead to a reduction in health insurance coverage, President Yoon Suk Yeol mentioned a second plan to reform health insurance. Regarding the direction of health insurance reform at the first state affairs inspection meeting at the Blue House guesthouse on the 15th, President Yoon said, "It means that we will eli
- Policy
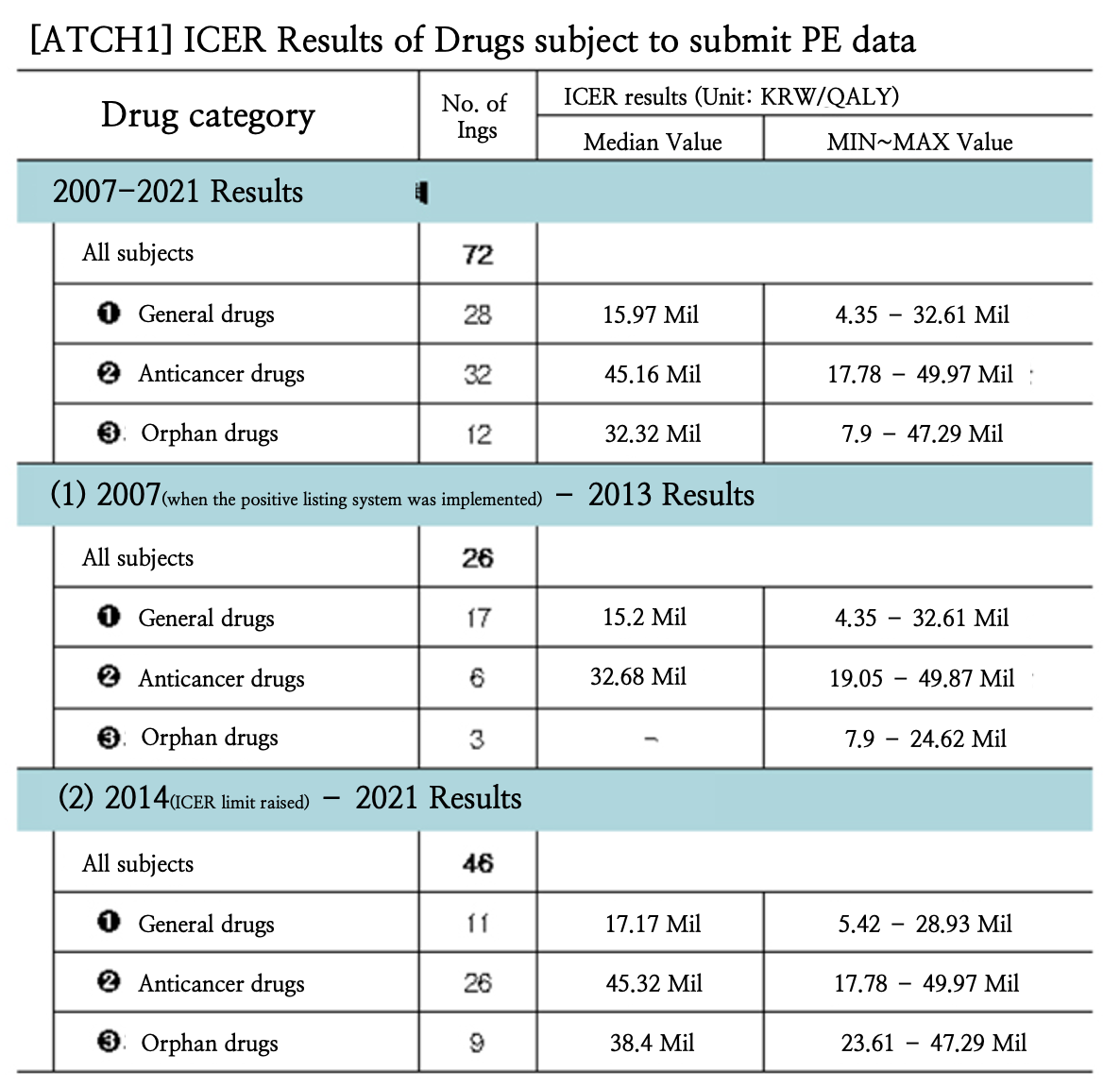
- ICER of general drugs KRW 15.97 mil for the past 15 yrs
- by Lee, Tak-Sun Dec 19, 2022 04:35am
- The median ICER (Incremental Cost-Effective Ratio) value of general drugs from 2007 to 2021 was KRW 15.97 million in Korea. The ICER value of anticancer drugs was KRW 45.16 million, and rare diseases KRW 15.97 million in the same period. This was the first time that the ICER results were disclosed, and the disclosed results are expected to be
- Policy
- Pfizer Cibinqo, re-applied to the HIRA
- by Lee, Tak-Sun Dec 19, 2022 04:35am
- Pfizer Cibinqo, which aims to pay for atopic dermatitis indications as a JAK inhibitor, is being paid later than expected. It was expected to be deliberated by the Drug Benefit Evaluation Committee within the year after passing the HIRA Drug Benefit Standards Subcommittee in August, but it is expected to take some time for the salary to be co