- Policy
- How did Zerbaxa get recognized for its benefit?
- by Lee, Tak-Sun Oct 24, 2022 06:08am
- There is an item that Rep. Choi Jae-hyung of the People's Power, who has been criticized by the pharmaceutical industry for insisting on lowering generic drug prices during the parliamentary audit, is praising his salary registration. It is the super antibiotic Zerbaxa. At the HIRA parliamentary audit held on the 13th, he said, "200 to 300 pe
- Policy
- Will Gilead reclaim its throne in the HCV drug market?
- by Lee, Tak-Sun Oct 24, 2022 06:08am
- Fierce competition is being expected in the hepatitis C (HCV) treatment market with the launch of Gilead’s new treatment which is priced at a lower level than the current market leader. With the new product, Gilead is aspiring to reclaim the former glory that it had enjoyed dominating the market with Sovaldi. According to industry so
- Policy
- Severe AD treatments unlikely to be reimb this year
- by Lee, Tak-Sun Oct 21, 2022 05:48am
- It seems that reimbursement extensions for severe atopic dermatitis treatments like Dupxient to benefit pediatric and adolescent patients will only be possible after next year. This is because the current reimbursement review process in progress would render reimbursements within the year difficult. This news was revealed in the writte
- Policy
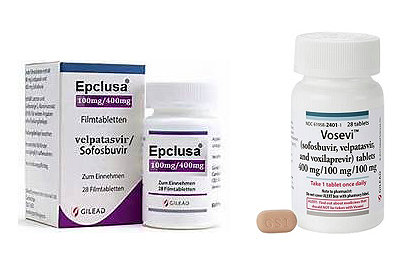
- Epclusa, a hepatitis C tx, costs 117,030 won
- by Kim, Jung-Ju Oct 21, 2022 05:48am
- Gilead Sciences Korea's Sofosbuvir/Velpatasvir-based chronic hepatitis C drugs Epclusa and Vosevi are expected to be listed on the 1st of next month at insurance drug prices of 117,000 won and 128,800 won, respectively. The government and insurance authorities expect to reduce health insurance finances as prices are agreed to be cheaper than alt
- Policy
- The head of the MFDS actively agrees with the opinion
- by Lee, Hye-Kyung Oct 21, 2022 05:47am
- Oh Yu-kyung, head of the Ministry of Food and Drug Safety, expressed her intention to actively agree to the introduction of active ingredients. Health and Welfare Minister Cho Kyu-hong avoided answering, saying that he would discuss various measures with the Ministry of Food and Drug Safety to ensure a smooth supply and demand of medicines in
- Policy
- Xtandi’s use expanded... reimbursement standard changed
- by Lee, Tak-Sun Oct 20, 2022 06:02am
- The scope of use for the prostate cancer treatment Xtandi soft capsule (enzalutamide, Astellas Korea) will be expanded. Although the drug was applied reimbursement only when other drugs that suppress androgen production cannot be used, reimbursement will be applied regardless of such status starting next month. On the 18th, the Health In
- Policy
- All targets for the benefit reassessment will be negotiated
- by Lee, Tak-Sun Oct 19, 2022 05:48am
- All six active ingredients-related companies that re-evaluated their benefit adequacy with the HIRA this year will meet at the negotiating table with the NHIS. In particular, the components for which the benefit maintenance has been decided will also be negotiated with the NHIS. Streptokinase and Streptodornase will negotiate the recovery
- Policy
- Is it possible to benefit from the K-CAB 4th indication?
- by Lee, Tak-Sun Oct 17, 2022 10:52pm
- Attention is focusing on whether HK inno. N's new drug K-CAB for gastroesophageal reflux disease will succeed in providing additional indications. Currently, K-CAB is being reimbursed to treat erosive and non-erosive gastroesophageal reflux disease and gastric ulcer. In March 2019, K-CAB, which was first applied to the treatment of erosive an
- Policy
- PVA, financial resources to register new drugs, not penaltie
- by Lee, Jeong-Hwan Oct 17, 2022 10:52pm
- Regarding the criticism that PVA imposes penalties on pharmaceutical companies that produce drugs with high public demand, the Ministry of Health and Welfare countered that it is a system that contributes to improving patient accessibility through new drug insurance. The Ministry of Health and Welfare also said that it is impossible to tem
- Policy
- “No considerations made to protect generics” at NA audit
- by Lee, Jeong-Hwan Oct 17, 2022 10:52pm
- After the Ministry of Health and Welfare revealed its plan to lower the price of generics step by step at the 2022 NA Audit, the domestic pharmaceutical industry has been expressly expressing their displeasure on how pro-government the regulation is and criticized how the plan does not consider the realities of the industry. The indust