- Policy
- The price gap between Memantine products is more than 2.5x
- by Lee, Tak-Sun Jun 7, 2022 06:04am
- The price difference between Memantine producst was more than 2.5 times. This is because if there are more than 20 generics depending on the price calculation formula, new products are listed at 85% of the lowest price. Memantine preparations have originally had a large price gap between products, but the gap is widening according to this pri
- Policy
- Price Negotiation for One-shot Treatment Zolgensma
- by Lee, Tak-Sun Jun 7, 2022 06:04am
- Zolgensma of Novartis, which passed the Drug Reimbursment Evaluation Committee of the HIRA on the 12th of last month, began negotiations on drug prices to register health insurance benefits. Given that Kymriah of the same company, which has attracted attention as a once-in-a-lifetime drug, succeeded in being reimbursed two months after passin
- Policy
- Zerbaxa passed evaluation committee after 4 yrs of challenge
- by Lee, Tak-Sun Jun 3, 2022 06:36am
- MSD Korea's antibiotic drug Zerbaxa has been recognized for its benefit adequacy by the HIRA Drug Reimbursment Evaluation Committee. As a result, it is likely to be listed on health insurance benefits through NHIS negotiations. The HIRA released the results of the 6th Drug Reimbursment Evaluation Committee review on the 2nd, and determined
- Policy
- Lioresal was newly listed
- by Kim, Jung-Ju Jun 2, 2022 05:59am
- With the new registration of 10mg/5mL of the skeletal muscle relaxant, the standard was newly established this month. Jakabi 5mg, which belongs to an anti-malignant tumor drug, has been clearly set in accordance with the change in permission from the MFDS. The MOHW announced that it partially revised the "details on the criteria and method
- Policy
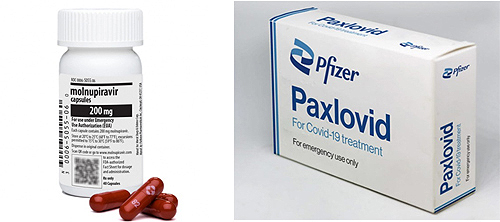
- New guidelines for Lagevrio & Paxlovid have been released
- by Kim, Jung-Ju Jun 2, 2022 05:58am
- New clinical guidelines for Lagevrio and Paxlovid's administration of COVID-19 patients have been released. Lagevrio considers medication if it is difficult to use other treatments among confirmed patients with mild and severe symptoms over the age of 18, and Paxlovid considers patients aged 12 or older who weigh more than 40kg. Eight m
- Policy
- The GMP reinforcement bill passed the plenary session
- by Lee, Jeong-Hwan Jun 2, 2022 05:58am
- Sustainable Support for New Drug Development Pharmaceutical Companies Increasing Punishment for Violations of GMP A bill to extend special cases for innovative pharmaceutical companies and a bill to significantly strengthen the level of GMP regulatory management compared to the previous one passed the plenary session of the National Assemb
- Policy
- 22nd new homegrown drug Acelex adds side effect after PMS
- by Lee, Hye-Kyung May 31, 2022 06:06am
- Crystal Genomics’ ‘Acelex Tab (polmacoxib)’ that was approved as the 22nd homegrown new drug in Korea will be adding ‘strokes, etc’ to its list of adverse events. The Ministry of Food and Drug Safety announced that it had completed the opinion review for the Change in Acelex's Indications based on Reevaluation Results (draft) and wil
- Policy
- President Visited KDCA to Strengthen Bio-Health Investment
- by Lee, Hye-Kyung May 31, 2022 06:05am
- President Yoon Seok-yeol visited the KDCA in Osong at 2 p.m. today (26th) to emphasize strengthening investment in key infrastructure in the bio-health industry. On this day, President Yoon discussed with experts how to promote scientific quarantine to prepare for the COVID-19 re-pandemic in autumn and winter at the KDCA Emergency Situation C
- Policy
- Will Dukarb Plus next month rise as Boryung’s savior?
- by Lee, Tak-Sun May 30, 2022 05:42am
- A product that uses the antihypertensive ingredient fimasartan (Kanarb) that Boryung Pharmaceutical developed will be released next month. The product, Dukarb Plus, is a fixed 3-drug combination of fimasartan, amlodipine, and hydrochlorothiazide. Dukarb Plus will be listed for insurance benefit as of June 1st. The release of Dukarb Plus
- Policy
- Chong Kun Dang faces Hanmi due to Rivaroxaban 2.5mg
- by Lee, Tak-Sun May 29, 2022 05:14pm
- Chong Kun Dang anticoagulant Rivaroxaban 2.5mg will be listed as the second generic drug after Hanmi. Since Chong Kun Dang has shown extraordinary affection for anti-coagulation oral new drugs, it is expected that it will also focus its efforts on low dose Rivaroxaban products. According to the industry on the 22nd, Riroxia capsules 2.5mg