- Policy
- Daewoong’s P-CAB drug Fexclu receives conditional approval
- by Lee, Tak-Sun May 16, 2022 06:14am
- Daewoong Pharmaceutical’s P-CAB class gastroesophageal reflux disease (GERD) treatment ‘Fexclu’ has received conditional approval from the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee. The committee decided reimbursement was appropriate for the drugs if the company accepts a price below the apprais
- Policy
- MOHW holds a launching meeting of the pharmaceutical team
- by Kim, Jung-Ju May 13, 2022 05:46am
- The MOHW (Minister Kwon Deok-cheol) announced at 3 p.m. on the 12th that it will hold a launching meeting of the mid- to long-term strategic planning team for the pharmaceutical industry to establish the "third comprehensive plan for fostering and supporting the pharmaceutical industry." The government shall prepare a five-year comprehensive
- Policy
- Vaccine consultative body launched
- by Kim, Jung-Ju May 13, 2022 05:45am
- Representative vaccine companies in Korea will launch a "Vaccine consultative body" to establish vaccine sovereignty and enhance international competitiveness, and actively cooperate with the aim of strengthening R&D capabilities in the industry and promoting vaccine commercialization. The MOHW (Minister Kwon Deok-cheol) and the Vaccine Pr
- Policy
- Will Ho-Young Chung be able to enter Cabinet?
- by Lee, Jeong-Hwan May 12, 2022 06:07am
- With the inauguration of the new administration on the 10th, whether the Health and Welfare Minister nominee Ho-Young Chung will be able to enter President Yoon’s Cabinet is now at a crossroads. Industry experts expect that the nominee will be appointed today (12th) after the Minister of Economy and Finance Choo Kyung-ho starts his role
- Policy
- Evusheld will be included in Govnt's supplementary budget
- by Lee, Jeong-Hwan May 12, 2022 06:06am
- The government is setting out to secure a supplementary budget for the introduction of ‘Evusheld (tixagevimab, cilgavimab),’ an antibody combination treatment used to prevent COVID-19 infection in the immunocompromised that see a low effect from vaccinations. With the National Assembly also agreeing on the need to introduce Evusheld, it
- Policy
- “Dualized RWD collection for Kymriah is inefficient”
- by Lee, Tak-Sun May 11, 2022 05:55am
- With the registration of the ultra-expensive one-shot treatment Kymriah, the opinion was raised that a plan should be prepared to ensure the efficiency of the system by standardizing the dualized real clinical data (RWD) collection required by different institutions. That the separate collection of data by the Ministry of Food and Drug S
- Policy
- Reblozyl was approved after 1 yr of designation of rare drug
- by Lee, Hye-Kyung May 11, 2022 05:55am
- Reblozyl 25mg, a treatment for patients with beta-Mediterranean anemia, was officially approved for items a year after designation as a rare drug. The MFDS approved Reblozyl applied by BSM Pharmaceutical Korea. Reblozyl is equipped with▲ the treatment of adult beta-Mediterranean anemia patients who need red blood cell transfusion as an
- Policy
- President Yoon said he was grateful to the people
- by Lee, Jeong-Hwan May 11, 2022 05:55am
- President Yoon Seok-yeol said on the 10th, "I am deeply grateful to the medical staff for their respect and dedication to the people who have endured great pain in the process of overcoming the COVID-19 pandemic over the past two years." At the inauguration ceremony of the 20th president held in front of the main building of the National Asse
- Policy
- Only 40% of asthma pts undergo Pulmonary function testing
- by Lee, Tak-Sun May 6, 2022 05:46am
- Although it is recommended to take pulmonary function test at least once a year to manage diseases in asthma patients, only less than half of all patients are tested. The HIRA announced on the 3rd that it will analyze and disclose the results of the 2020 (8th) asthma adequacy evaluation to mark World Asthma Day. As a result of the evaluation,
- Policy
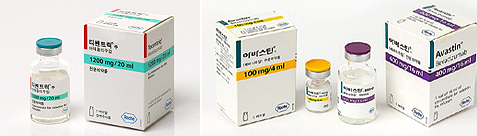
- New cost of Tecentriq is 2,271,109 won
- by Kim, Jung-Ju May 2, 2022 06:02am
- Tecentriq, an immuno-cancer drug from Roche Korea, will be expanded from next month due to a combination treatment with Avastin. The new insurance drug price is 2,271,109 won per 20mL for Tecentriq, 218,782 won per 100mg for Avastin, and 712,098 won for Avastin 400mg. The MOHW announced that the "Amendment to the Pharmaceutical Benefit