- Policy
- Combi of HBP+ Hyperlipidemia will be released again
- by Lee, Tak-Sun Apr 29, 2022 05:41am
- As Chong Kun Dang, Yuhan Corp., and GC Pharma, one of the top three traditional pharmaceutical companies in sales, are set to launch a combination of high blood pressure + hyperlipidemia, the market is expected to lead the prescription trend in the future. Currently, the market is led by improved complexes developed by Boryung, Hanmi Pharmace
- Policy
- Reviewing the removal of outdoor masks
- by Lee, Jeong-Hwan Apr 28, 2022 06:06am
- The presidential transition committee announced its position to examine the COVID-19 situation in late May, a month after the launch of the new government, and then decide to release the outdoor mask. The new government plans to promote quarantine policies based on scientific evidence and prepare governance to respond to infectious disease
- Policy
- The transition team operates biohealth regulatory sandboxes
- by Kang, Shin-Kook Apr 27, 2022 06:03am
- The presidential transition committee established the Pharmaceutical Bio Innovation Committee, which was the pledge of Yoon Seok-yeol, and presented deregulation through biohealth regulatory sandboxes as a policy task. Baek Kyung-ran, a member of the transition team's social welfare division, said on the 25th that he will recognize the biohea
- Policy
- Betamethasone's permission change is expected
- by Lee, Hye-Kyung Apr 27, 2022 06:03am
- As a result of reviewing safety information on drugs containing the skin disease treatment Betamethasone, precautions for use such as Pheochromocytoma are expected to be added. The MFDS' Pharmaceutical Safety Evaluation Division prepared a change (draft) of permission based on the results of the European Medicines Agency's review of safety
- Policy
- Phase III for development of Alecensa & Keytruda
- by Lee, Hye-Kyung Apr 26, 2022 06:11am
- Roche Korea and MSD Korea are accelerating clinical trials to develop combination therapy for non-small cell lung cancer treatments. On the 22nd, the MFDS approved phase 3 clinical trials of MSD's Keytruda and Keytruda SC and Roche's Alecensa. All of these pharmaceutical companies begin clinical trials to confirm the safety and effectiv
- Policy
- Exclude drugs that have increased due to COVID-19 from PVA
- by Lee, Tak-Sun Apr 25, 2022 06:08am
- It is reported that the pharmaceutical industry plans to officially propose to the government to exclude respiratory treatments that have increased their use due to the increase in COVID-19 patients from PVA. It is asked to reflect that the explosive increase in the use of the drug is inevitable due to the increase in the number of patients w
- Policy
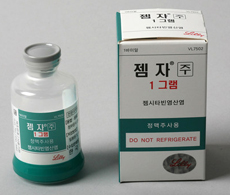
- Boryung’s Gemzar switches to domestic production
- by Lee, Hye-Kyung Apr 25, 2022 06:07am
- In 2 years since Boryung Pharmaceutical acquired the domestic rights for Lilly’s anticancer treatment ‘Gemzar (gemcitabine HCl)’ in Korea, the company switched all its items to domestic productions. According to the Ministry of Food and Drug Safety, Boryung Pharmaceutical withdrew its import license for ‘Gemzar’ on the 21st, and swit
- Policy
- Chong Kun Dang salt modified Entresto will be released soon
- by Lee, Hye-Kyung Apr 25, 2022 06:07am
- The market launch of Sacubitril/Valsartan Calcium, developed by Chong Kun Dang is imminent. According to the pharmaceutical industry on the 21st, Chong Kun Dang filed an application with the MFDS for permission for the drug developed by changing Valsartan Sodium, the main ingredient of Novartis' chronic heart failure treatment Entresto.
- Policy
- Expansion of benefit standards such as Tenofovir/Baricitinib
- by Kim, Jung-Ju Apr 24, 2022 06:33pm
- As the scope of benefit of registered drugs such as chronic hepatitis B oral drug Vemlidy expands, the benefit standards for these drugs will also be expanded and changed. In addition, the standards for Baricitinib PO such as oluminant 2mg will be expanded to patients with chronic severe atopic dermatitis. The MOHW unveiled the "revise
- Policy
- Expanding the use of Paxlovid with underlying dz over 12 yrs
- by Lee, Jeong-Hwan Apr 20, 2022 06:05am
- The government said it is considering expanding the scope of the prescription for Paxlovid, an oral treatment for COVID-19, to "underlying patients aged 12 or older." However, considering the fact that there are side effects, it is said that various decision-making processes such as collecting opinions from experts should be carried out. T