- Policy
- It will not be easy to reflect generic positive list system
- by Lee, Tak-Sun Apr 6, 2022 06:05am
- The NHIS has completed a study of external services that only provides a positive list of excellent generics, but it is not expected to be easy to reflect the policy. This is because the research was promoted by the current government and it is still unreasonable to lead to policies. On the 31st of last month, the NHIS registered a "resear
- Policy
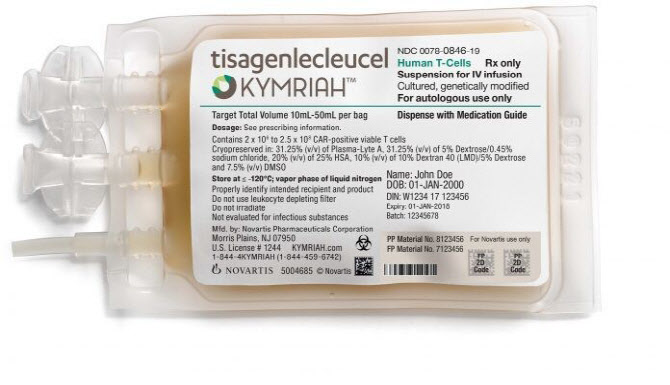
- Kymriah has been listed, hope of cure
- by Lee, Jeong-Hwan Apr 5, 2022 05:57am
- Patients immediately welcomed the confirmation of health insurance benefits of the acute lymphocytic leukemia and lymphoma CAR-T treatment Kymriah (Tisagencleucel). The patients urged the transition committee of the next presidential post and Yoon Seok-yeol to quickly register new drugs directly related to life as health insurance benefits
- Policy
- Phase 3 of Jetema the toxin will be conducted in Korea
- by Lee, Hye-Kyung Apr 4, 2022 06:07am
- Jetema the toxin 100U, which has been approved for export botulinum toxin, will conduct phase 3 clinical trials in Korea. The MFDS recently approved Jetema the toxin for a "multi-organization, double-blind, random assignment, parallel design, noninferiority trials, active control groups, and phase 3 clinical trials" for JTM201. The clinica
- Policy
- 20 ultra-high-priced drugs over ₩5 mil sold in Korea
- by Lee, Tak-Sun Apr 4, 2022 06:07am
- Survey results have shown that 20 high-priced drugs over &8361;5 million are approved for reimbursement 10 years after, Soliris, which had been then the most expensive drug in the world, was listed for reimbursement in Korea With ‘Kymriah,’ the one-shot treatment that was listed for reimbursement on April 1st, recording the highest pri
- Policy
- Based on PVA exclusion, the arithmetic average is 90%
- by Lee, Tak-Sun Apr 4, 2022 06:07am
- Drugs subject to PVA with an arithmetic average of less than 90% of the same product group are excluded. Previously, only drugs below the arithmetic average were excluded, but the target was further narrowed to less than 90%. Products with annual claims of less than 2 billion won are also excluded from PVA drugs. Previously, products worth
- Policy
- To commercialize innovative new drugs/supply essential drugs
- by Lee, Jeong-Hwan Apr 3, 2022 04:25pm
- The MFDS reports discuss ways to become a bio-health powerhouse The Presidential Acquisition Committee Yoon Seok-yeol and the MFDS agreed to systematically support the commercialization of high-tech and innovative medical products and establish a stable supply environment for rare essential drugs with low profitability. The transition com
- Policy
- The MFDS released a national lot release of Comirnaty
- by Lee, Hye-Kyung Mar 31, 2022 04:29pm
- The MFDS (Director Kim Kang-rip) announced on the 29th that it has released 299,000 doses of Pfizer's Comirnaty 0.1mg/mL (for 5-11 years old) in Korea. The MFDS conducted Comirnaty test and reviewed the manufacturing and test data of the manufacturer, and confirmed the effectiveness, safety, and quality, and decided to release the national
- Policy
- Roche RET target anticancer drug Gavreto, approved in Korea
- by Lee, Hye-Kyung Mar 31, 2022 04:23pm
- Roche's non-small cell lung cancer treatment Gavreto (Pralsetinib)"has obtained an item license in Korea. The MFDS approved Gavreto 100mg on the 29th. The drug was recognized for its effectiveness in the treatment of ▲RET (RET) fusion-positive local progression or metastatic non-small cell lung cancer adult patients and ▲ systemic therap
- Policy
- Reimbursement priorities in ultra-high-priced one-shot Txs
- by Lee, Tak-Sun Mar 31, 2022 05:57am
- With the reimbursement imminent for the ultra-high-priced one-shot treatment Kymriah, the National Health Insurance Service is preparing to conduct research on the performance evaluation of the risk-sharing agreement (RSA) and its mid-to-long-term development direction. The research will be exploring ways on the development direction of
- Policy
- Multidrug-resistant ‘Zavicefta’ applies for approval
- by Lee, Hye-Kyung Mar 29, 2022 05:54am
- Pfizer Korea has applied for the approval of its important treatment for severe gram-negative bacterial infection, ‘Zavicefta.’ Zavicefta, which received marketing authorization in 2016. is a combination drug that contains the third-generation ceftazidime. According to industry officials on the 28th, Pfizer Korea recently submitted a