- Policy
- There were no applications for Ultomiris·Soliris benefits
- by Lee, Hye-Kyung Jan 6, 2022 06:09am
- In November last year, it was confirmed that there was no pre-application for Ultomiris and Soliris benefits for new patients with paroxysmal night hemoglobin (PNH). There were only two applications for pre-approval of Soliris and one application for re-examination approval for new patients with atypical hemolytic uremic syndrome (aHUS), all
- Policy
- Companies obtain generic for exclusivity in proportion
- by Lee, Tak-Sun Jan 6, 2022 06:08am
- Among pharmaceutical companies that use generic for exclusive in the licensed patent linkage system that took effect in 2015, it was found that companies with large sales are more likely to obtain generic for exclusivity. As a result, it was suggested that small and medium-sized pharmaceutical companies need more support such as consulting.
- Policy
- Request for non-reimbursed use of Pahtension in Loop's pts
- by Lee, Hye-Kyung Jan 6, 2022 06:08am
- Application for Pahtension 20mg was rejected for non-reimbursed patients with systemic erythema lupus accompanied by immunosuppressants, vasculitis that does not improve even with antibiotics, and peripheral ulcers. The HIRA is receiving applications in advance for use exceeding the MFDS' permission to prevent the use of drugs that lack medic
- Policy
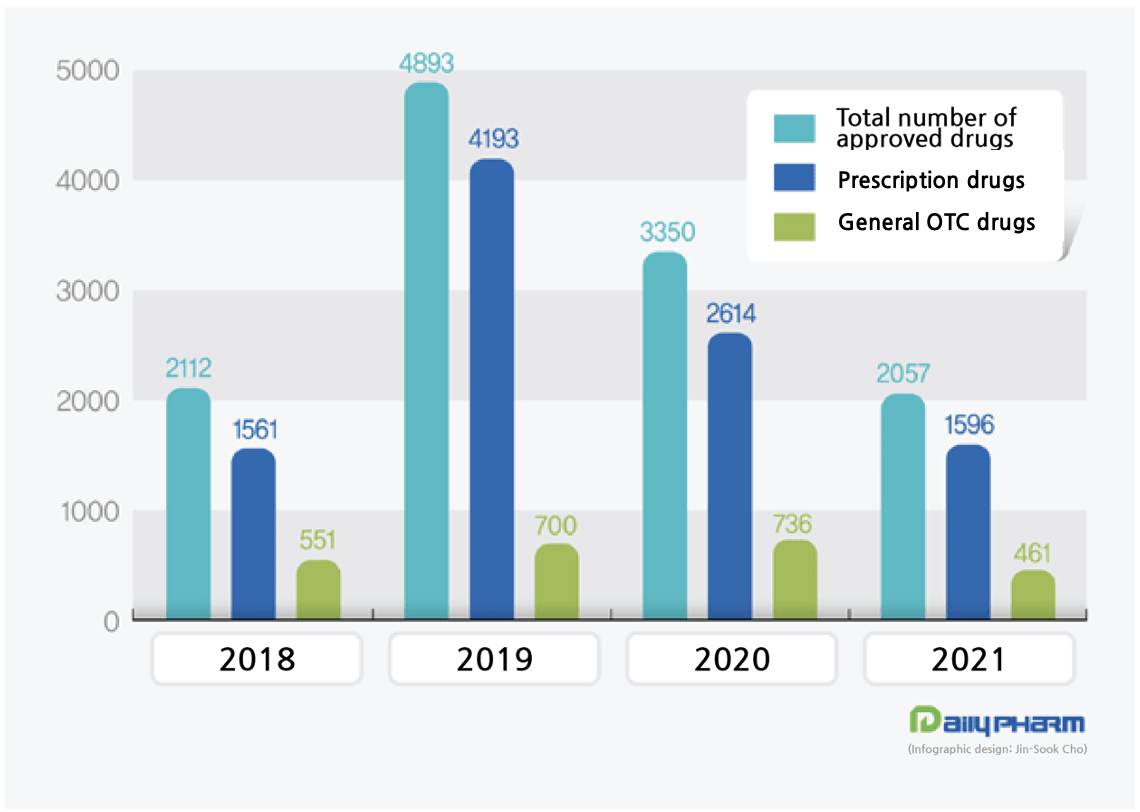
- ‘Lowest-ever’ number of drugs approved in 4 years
- by Lee, Tak-Sun Jan 5, 2022 05:59am
- The year 2021 is likely to be remembered as the year of reduced marketing authorizations for drugs. This was greatly influenced by the changes in the market and the new regulations that were introduced last year. In particular, the pricing penalty imposed on indirect bioequivalence tested drugs and the restrictions set on consigned bioequivalenc
- Policy
- Combination drugs of PPI+ antacid have also been released
- by Lee, Tak-Sun Jan 5, 2022 05:57am
- In the anti- ulcer drug market, combination drugs of PPI+ antacids have been released. This time, it is a drug that combines Rabeprazole and sodium bicarbonate. The PPI+ antacids, which started with Chong Kun Dang's Eso Duo (Esomeprazole Magnesium Trihydrate+Sodium Bicarbonate), continues to develop new products. The MFDS approved Youngjin
- Policy
- Recovery Action held by the Legislation/Judiciary Committee
- by Lee, Jeong-Hwan Jan 4, 2022 05:56am
- The recovery system of drug prices bill, which drew attention from the government and domestic and foreign pharmaceutical companies, has been held by the National Assembly's Legislation and Judiciary Committee and is in trouble to deal with it. As it was excluded from the Legislation and Judiciary Committee agenda again during the extraordin
- Policy
- President Moon "will make all efforts to return to normalcy"
- by Lee, Jeong-Hwan Jan 4, 2022 05:56am
- During his 2022 New Year’s address, President Moon Jae-in expressed his gratitude and respect to the medical professionals and personnel working to contain and prevent COVID-19 and restressed the excellence of Korea’s disease control and prevention system. The president also expressed his ambition to achieve complete recovery of the peo
- Policy
- Minister Kwon said he would develop vaccines and txs
- by Kim, Jung-Ju Jan 4, 2022 05:55am
- Minister of Health and Welfare said he will foster the biohealth industry by investing in building a global bio-human resource training hub, developing vaccines and treatments, creating a "K-Global" vaccine fund and revitalizing the use of domestic medical devices in 2022. Minister Kwon issued a New Year's address today (31st) and explained t
- Policy
- Additional contracts for COVID-19 PO tx are under discussion
- by Kim, Jung-Ju Jan 4, 2022 05:55am
- The government is pushing for additional purchases of oral COVID-19 treatments separately from existing contracts.The KDCA announced its 2022 work plan today (30th) under the theme of "COVID-19 Disease Control and Prevention Response and National Health Support." Authorities plan to strengthen severe prevention by introducing oral treatmen
- Policy
- Changes in Benefit standards such as diabetes drugs
- by Kim, Jung-Ju Dec 31, 2021 05:50am
- The general principles of diabetes solvents and psychotropic drugs and some of Soliris (Eculizumab)'s detailed recognition criteria and methods for benefits change. Testosterone undecanoate, such as Andriol Testocaps Soft Cap, a male hormone drug, is classified by item, and detailed recognition criteria and methods are deleted. The MOHW an