- Policy
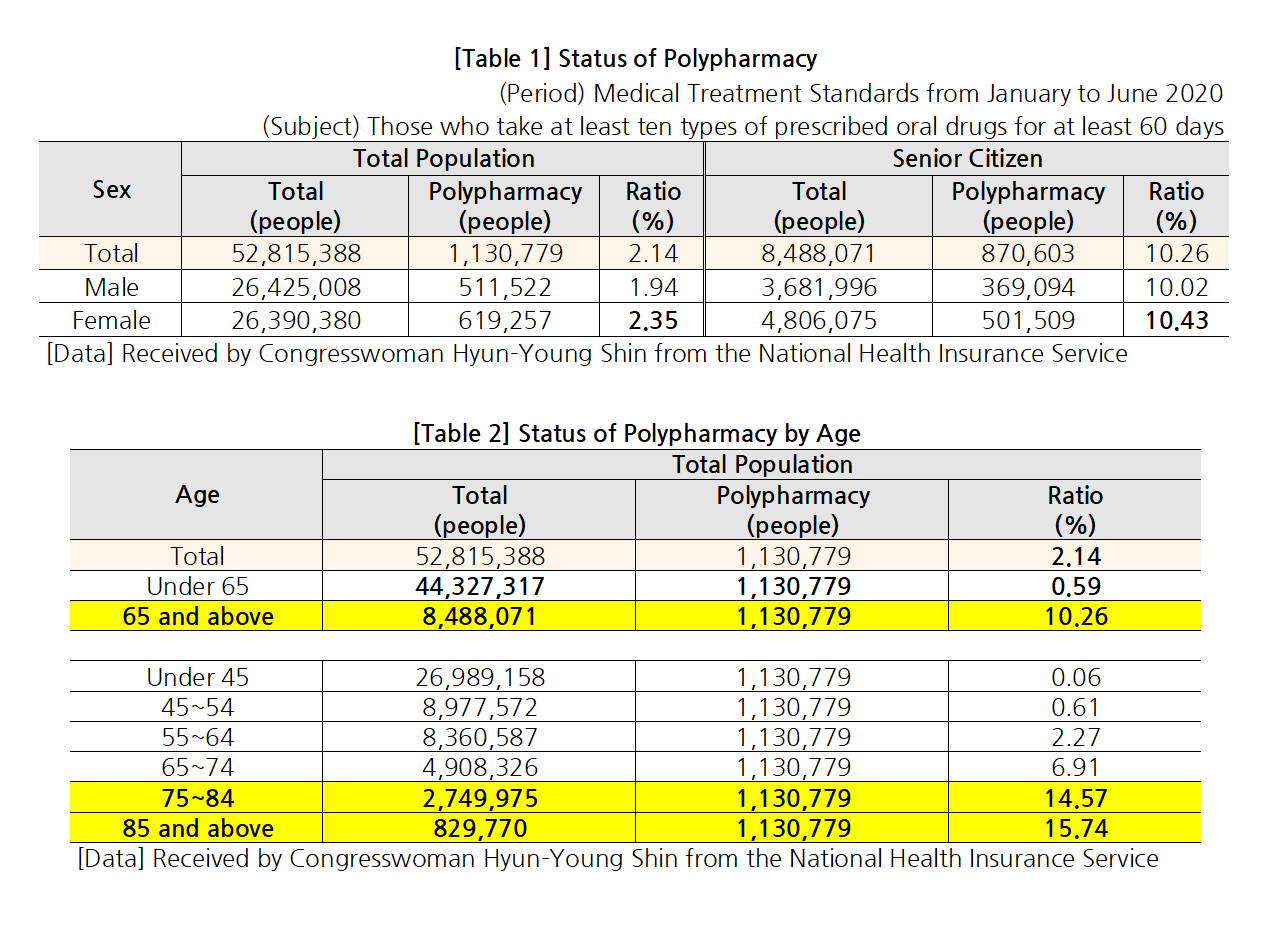
- “Elderly polypharmacy rate Korea 70% vs OECD countries 48%"
- by Lee, Jeong-Hwan Oct 6, 2021 06:06am
- Statistics showed that a high rate - 70.2% - of older adults (aged 75 or over) in Korea chronically take ‘more than 5 drugs for over 3 months.’ The average of 7 OECD countries other than Korea that submitted the same data was only 48%. In other words, concerns over the current polypharmacy status of elderly patients in Korea have been r
- Policy
- The patient died due to the delay in Kymriah benefits
- by Lee, Jeong-Hwan Oct 6, 2021 06:06am
- Korea leukemia patients organization launched a one-man protest, urging the first C-ART treatment Kymriah (Tisagencleucel)'s fast track. The Korea Leukemia Association held a press conference in front of the National Human Rights Commission of Korea at 10 a.m. on the 1st and announced that it submitted a petition to the National Human Rig
- Policy
- Boryung's Dukarb Plus is about to be commercialized
- by Lee, Tak-Sun Oct 5, 2021 05:58am
- It was found that commercialization of the high blood pressure combination drugs including Boryung's Fimasartan is imminent. If this product is approved, a total of four products will form treatment options in the hypertension treatment lineup leading to Kanarb-Kanarb Plus-Dukarb. Through this, it is expected that the diversity of patient
- Policy
- Rate of reimbursement expansions is falling?
- by Lee, Hye-Kyung Oct 5, 2021 05:56am
- To the criticism that the rate of reimbursement extensions for anticancer drugs have been falling, the Health Insurance Review and Assessment Service had pointed to the increased number of high-priced drugs that are being covered with the RSA system. HIRA had explained that the increased number of drugs covered through the RSA system sinc
- Policy
- Kymriah's fast track is needed
- by Kim, Jung-Ju Oct 5, 2021 05:56am
- Patient groups objected to the decision of the Cancer Drugs Benefit Appeal Committee on hold against Kymriah (Tisagenleclecel), which drew attention to insurance benefits as a cell therapy and a "one-shot treatment." This is because it is a new drug that is directly related to life for leukemia patients, and even though they have to be paid i
- Policy
- Botulinum toxin by fraudulent means can be revoked
- by Lee, Jeong-Hwan Oct 1, 2021 06:08am
- Regulations on handling and management, such as reporting high-risk pathogens such as botulinum toxin and anthrax, will be strengthened, and permission for bioterrorism infectious disease pathogens licensed by fraudulent methods will be revoked. On the 28th, Baek Jong-heon (Busan Geumjeong-gu), a member of the National Assembly's Health an
- Policy
- Domestic new drugs shouldn't be discriminated against
- by Lee, Jeong-Hwan Oct 1, 2021 06:08am
- Problems with the PVA system are expected to be discussed during this year's parliamentary audit of the National Assembly's Health and Welfare Committee. The domestic pharmaceutical community criticized that it is too harsh for the government to unilaterally decide on a drug price cut without acknowledging the innovation of new domestic drugs
- Policy
- Pts can participate in Phase III tx for terminal cancer
- by Lee, Tak-Sun Sep 30, 2021 05:57am
- The MFDS announced on the 29th that it has revised and distributed the "Guidelines for applying rapid screening of medicines" so that even early cancer patients can participate in clinical trials, considering the difficulty of recruiting large-scale phase 3 clinical trials for terminal cancer patients. However, even in the early stages of the
- Policy
- 24 new drugs receive ₩177.9 billion benefit this year
- by Kim, Jung-Ju Sep 30, 2021 05:56am
- A total of 24 new drugs were newly listed or expanded reimbursement standards by this month, improving patient accessibility. Among new drugs that were already listed, reimbursement standards for 4 products were extended, improving coverage. Although only 10,000 patients in Korea will benefiting from the extension, the result could be interp
- Policy
- Will a treatment for resistant hypertension be released?
- by Lee, Tak-Sun Sep 29, 2021 05:54am
- Attention is focused on whether a treatment for resistant hypertension that cannot be controlled with existing drugs, that is in the final stages of its clinical trial, will succeed in commercialization. The drug is firibastat, that is developed by Quantum Genomics. Dong Wha Pharmaceuticals owns exclusive commercialization rights to supp