- Policy
- Rekirona are expanded to 50 years of age or older
- by Lee, Tak-Sun Sep 24, 2021 05:56am
- The MFDS approved the official approval based on the results report of phase 3 clinical trials of Celltrion's COVID-19 antibody treatment Rekirona, which completed phase 3 global submitted by the company. It has expanded the subject of administration. However, mild patients were not included. The MFDS announced on the 17th that it had careful
- Policy
- Pfizer voluntarily recalls Champix 10 days after MFDS annc.
- by Lee, Tak-Sun Sep 23, 2021 05:44am
- Some lots of Pfizer’s leading smoking cessation treatment, Champix, will be voluntarily recalled. Although the drugs were not subject to recall under the MFDS announcement on the 7th, Pfizer opted to voluntarily recall its products as the drugs exceeded the MFDS's temporary release standards. The standards that the company applied for
- Policy
- Varenicline recalls were based on Pfizer's standards
- by Lee, Tak-Sun Sep 23, 2021 05:43am
- On why the recall and lot release standards were set differently for the smoking cessation treatment ‘varenicline,’ which was found to have nitrosamine impurities, is gaining attention. This difference in standards had allowed Pfizer’s Champix from being recalled. While announcing the N-nitroso-varenicline (NNV) impurity test results
- Policy
- Price for Kymriah needs a new management method
- by Lee, Hye-Kyung Sep 23, 2021 05:43am
- The NHIS continues to discuss finding a new drug price management method for the rapid registration of ultra-high-priced "one-shot treatments." However, he said he would consider the introduction of "post-registration evaluation" required by patient organizations as one of several alternatives, not the correct answer. The Guidelines for Calcu
- Policy
- Azelnidipine was approved in Korea
- by Lee, Tak-Sun Sep 17, 2021 05:56am
- Azelnidipine, which was approved in 2003 in Japan as a calcium channel blocker (CCB), was also approved in Korea. There was no previously approved finished product. The MFDS approved Azelnidipine 8mg of Introbiopharma on the 14th. Azelnidipine is a treatment for hypertension, a drug administered oral after breakfast once a day. Previous
- Policy
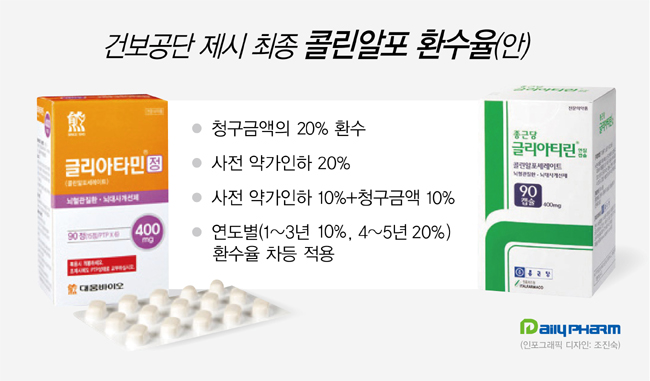
- 58 companies agree on the redemption of α- GPC benefits
- by Lee, Hye-Kyung Sep 17, 2021 05:56am
- Negotiations on the conditional return of benefits for clinical reevaluation of "Choline alfoscerate," a brain function improving agent that has been going on for about nine months, will end today (15th). The final result is that all 58 pharmaceutical companies with 123 Choline alfoscerate agreed to "return 20% of their health insurance claim
- Policy
- Will Jeil Pharm gain a hold over the varenicline market?
- by Lee, Tak-Sun Sep 16, 2021 05:59am
- Following the government’s measures to reduce impurities in smoking cessation drugs that contain varenicline, Jeil Pharamceutcial’s products, which satisfy the set standards, is expected to gain a hold over the smoking cessation treatment market for the time being. The Ministry of Food and Drug Safety has allowed the lot release of vare
- Policy
- Drug substances with impurities are collected voluntarily
- by Lee, Tak-Sun Sep 16, 2021 05:59am
- The recovery of antihypertensive drugs exceeding impurities is expanding. Following the Drug product, Drug substance began to recover. The MFDS announced on the 14th that it will voluntarily retrieve some of Kukjeon and Pharmacostech's Irbesartan's Drug Substance. Kukjeon and Pharmacostech start collecting four manufacturing number products e
- Policy
- 299 items were voluntarily withdrawn
- by Lee, Hye-Kyung Sep 15, 2021 06:12am
- The NHIS's self-evaluation came out that the "National Health Insurance Medical Care Benefit Standards" revised on October 8 last year prevented the registration at will. At the Korea Special Press Association briefing held on the 14th, Lee Sang-soo, executive director of insurance benefit, said, "As soon as the drug benefit list was regis
- Policy
- Controversy over the price of COVID PO of ₩900,000
- by Lee, Jeong-Hwan Sep 15, 2021 06:11am
- While the government, which judged that oral treatments are needed to shift the COVID-19 phase, is negotiating pre-purchase with global pharmaceutical companies, it is pointed out that drug prices will reach &8361;900,000. The government also acknowledges that the price of oral COVID-19 treatment is expensive, but if they have a therapeut