- Policy
- “Mooncare a success…37 million people saved ₩9 tril.
- by Lee, Jeong-Hwan Aug 13, 2021 05:57am
- President Moon Jae-in said the government will continue and accelerate its efforts to further broaden the NHI coverage in 2022 as in this year. Moon also stated that 37 million people in Korea were able to save a total of 9.2 trillion won in medical costs during the 4 years since Mooncare was implemented. Also, he personally announced
- Policy
- Regkirona's indication expansion has been requested
- by Lee, Tak-Sun Aug 13, 2021 05:57am
- Celltrion's COVID-19 antibody therapy Regkirona is pushing for an expansion of indications based on global phase 3. Regkirona was conditionally approved to submit a clinical trial result report for therapeutic confirmation in February and was used only in patients with mild to secondary COVID groups. Celltrion submitted a report on the result
- Policy
- With Ultomiris, 9 items require prior approval for reimb.
- by Lee, Hye-Kyung Aug 12, 2021 06:05am
- Improvements are expected to be made to the ‘pre-approval' system that may be actively used in the process of reimbursement listings for ultra-high-priced drugs. Starting with the stem cell transplantation procedure in 1992, the Health Insurance Review and Assessment Service (HIRA) has been operating the pre-approval system to deliberate in
- Policy
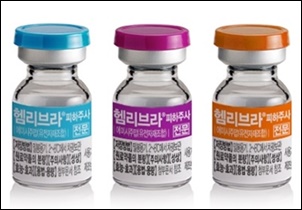
- A review of Hemlibra's benefit without ITI is under way
- by Lee, Hye-Kyung Aug 12, 2021 06:05am
- The HIRA is reviewing the benefit criteria for JW Pharma's hemophiliac injection Hemlibra (Emicizumab). Currently, patients aged 1 and older and under 12 years of age may be re-administered if they fail the Immune Tolerance Induction (ITI) or if they meet the ITI target criteria (Trial 2020-164, 2020. 6.1.), or if they are unable to try,
- Policy
- President called for stronger health insurance coverage
- by Kang, Shin-Kook Aug 12, 2021 06:04am
- President Moon called Yong-Ik Kim, President of NHIS and Sun-min Kim, Executive Director of the HIRA to discuss the results of measures to strengthen health insurance coverage and supplement the people's benefits at meeting with his senior secretaries on the 9th. President Moon ordered, "In case of not receiving medical benefits due to c
- Policy
- # of subjects in Phase 3 of SK's COVID vaccine is 93
- by Lee, Tak-Sun Aug 11, 2021 05:58am
- When looking at SK Bioscience's clinical plan for "GBP510," which entered phase 3 for the first time as a vaccine developed in Korea, the number of people tested in Korea was 93. This is only 2.3% of 3,990. The MFDS recommends that domestic test subjects recruit more than 10% of the total subjects for domestic development vaccines, which i
- Policy
- South Korea recovered Sartan products with AZBT impurities
- by Lee, Tak-Sun Aug 11, 2021 05:58am
- The recovery of Sartan medicines containing 'AZBT' has also begun in Korea. Three products containing Irbesartan, manufactured in 2021, were voluntarily recovered by related pharmaceutical companies. Starting in Canada in May, recovery of Sartan containing AZBT is spreading around the world to Europe and Asia. The MFDS announced on the 6th
- Policy
- Incentive-paid items for generic substitution fell to 12,757
- by Lee, Hye-Kyung Aug 11, 2021 05:58am
- 12,757 items were found to be eligible for incentives of 30% of the difference in drug prices if pharmacies dispense generic substitution, which is cheaper than prescription drugs. It decreased 48 items compared to the previous month. The HIRA recently guided the 'Billing Method of Drugs Subjected to Incentives for low-price generic substitu
- Policy
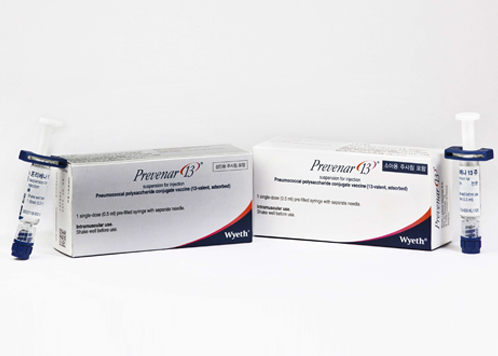
- Pfizer is working hard to introduce Prevenar-20 in Korea
- by Lee, Tak-Sun Aug 10, 2021 05:54am
- Pfizer, which supplies Prevenar-13, pneumococcal vaccine that ranks overwhelmingly at the top of the domestic market, is also working hard to introduce the next-generation vaccine. It started clinical trials for East Asians to introduce Prevenar-20, which was approved by the U.S. Food and Drug Administration (FDA) in June. Prevenar-20 h
- Policy
- Supply of Oseltamivir has declined 55% since COVID outbreak
- by Lee, Hye-Kyung Aug 10, 2021 05:54am
- After COVID-19 epidemic, the supply of respiratory medicine has dropped by nearly half. According to the HIRA's "Medicine Distribution Newsletter No. 1" published on the 5th, the supply of respiratory drugs in spring and winter since 2020 decreased by 40.6% compared to the same period last year, and the supply of Oseltamivir used to treat flu