- Policy
- The price of 7 Seretide products will be reduced
- by Kim, Jung-Ju Jul 5, 2021 05:53am
- The government won the suit. But there is a possibility of change depending on the company's decision. Insurance prices for 7 items of asthma treatment Seretide by GSK will be lowered from the 2nd of next month. The government won the lawsuit against the company to cancel the reduction of the price. If the company disagrees with the ruling, t
- Policy
- The reasons why Hemlibra's benefit was not recognized?
- by Lee, Hye-Kyung Jul 3, 2021 05:56am
- The HIRA's Central Review and Coordination Committee revealed specific reasons for not recognising benefit of JW Pharma's Hemlibra (Emicizumab). The HIRA released a June review of the Review and Assessment Committee on its portal on Thursday. There are five cases of deliberation, including Hemlibra, Soliris, ventricular assistive therapy,
- Policy
- Rationalizing ICER threshold requires various considerations
- by Kim, Jung-Ju Jul 1, 2021 05:56am
- Insurance authorities expect the evaluation results of the listed drugs that underwent quality reassessment on the reasonableness of their medical care benefit (re-evaluation of medical benefits of listed drugs) will be applied as early as October this year. This means that the results will pass the Health Insurance Policy Deliberative Comm
- Policy
- Targets for PVA in third quarter include Darzalex & Alunbrig
- by Lee, Hye-Kyung Jul 1, 2021 05:55am
- Janssen Korea's (Daratumumab) and Takeda Korea’s Alunbrig (Brigatinib) were included in the Price-Volume Agreement (PVA). Otsuka Pharmaceutical Korea's "Iclusig" and AstraZeneca Korea's "Impinji" (Dubalumab) were also subject to negotiations on drug prices due to increased usage. The NHIS recently introduced "PVA (Type Ka/Na) Monitorin
- Policy
- 2 indications deleted for choline alfoscerate, changes label
- by Lee, Tak-Sun Jun 30, 2021 05:56am
- Authorities have ordered companies to delete the No.2 and No.3 indication of the brain function enhancer choline alfoscerate, for which the clinical re-evaluations have started recently. Accordingly, the indication must be removed from the label from the 28th of next month. Also, the Ministry of Food and Drug Safety requested doctors and
- Policy
- Will Rosuvastatin OD be released?
- by Lee, Tak-Sun Jun 30, 2021 05:55am
- Attention is focusing on whether a treatment for hyperlipidemia, Rosuvastatin OD (Orally Disintegrated Tablet) will be released. Rosuvastatin OD was approved in Japan in 2016, but has not yet been released in Korea. According to industries on the 25th, BCWORLD Pharm recently applied for approval of Rosuvastatin OD to the MFDS. Astra
- Policy
- Janssen Vaccine for 10,800 people has been approved
- by Lee, Tak-Sun Jun 29, 2021 05:46am
- The MFDS said it approved the nation lot release on the 25th (Friday) for 108,800 people of "Covid-19 Vaccine Janssen" applied by Janssen Korea. Nation lot release is a system in which the state checks the quality of the vaccine once again before it is distributed on the market by comprehensively evaluating the MFDS' test results of the va
- Policy
- Leclaza was listed at ₩68,964 per tablet
- by Kim, Jung-Ju Jun 29, 2021 05:46am
- Leclaza 80mg (Lazertinib) made with domestic technology passed the final step to insurance benefits. Leclaza finally chose this track, considering its similar clinical utility to the alternative drug Tagrisso, but cheaper with the Risk Sharing Agreement (RSA) Refund and Expenditure Cap. The date will be started on July 1, which means that
- Policy
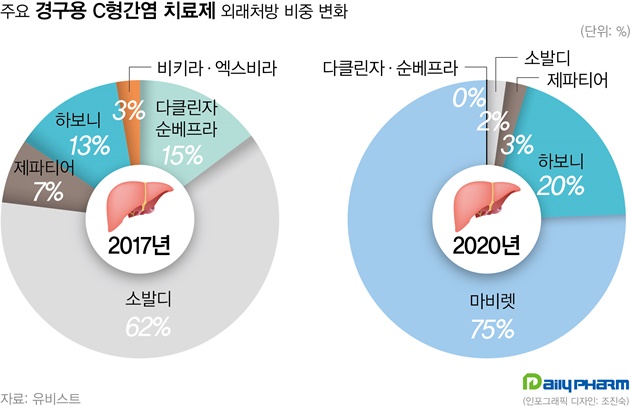
- Following Gilead's hepatitis C tx Epclusa, Vosevi was filed
- by Lee, Tak-Sun Jun 28, 2021 05:50am
- This year, hepatitis C drugs Epclusa and Vosevi have been filed by the MFDS in Korea. These items are expected to replace Sovaldi. According to the industry on the 27th, Gilead's Vosevi (Sofosbuvir/Velpatasvir/Voxilaprevir) has recently been filed and will begin screening in earnest. Vosevi is expected to be a Pangenotypic DAA preparation
- Policy
- 16 drugs including Venclexta receive reimbursement in 1H
- by Kim, Jung-Ju Jun 28, 2021 05:50am
- A total of 14 products were newly listed on the insurance benefit list to improve patient access in the first half of this year. Also, two drugs benefited from the reimbursement criteria expansion that was applied to already-listed new drugs. This expanded coverage is interpreted as a result of the government’s decision to flexibly apply covera