- Policy
- Gov will proactively improve system for severe psoriasis
- by Kim, Jung-Ju Jun 21, 2021 05:50am
- With a year left before the re-registration of special exemption of insurance calculation for severe psoriasis, the government, payer, and patient group gathered to discuss improvement. The issue discussed was that despite reimbursement approved for severe psoriasis drugs, patients are not being properly covered as the eligibility standards for
- Policy
- Yuhan's Raboni-D has been licensed
- by Lee, Tak-Sun Jun 21, 2021 05:50am
- Yuhan, which had a high dependence on sales for imported drugs, has recently been speeding up with the commercialization of new drugs such as Lazertinib and IMD. In particular, Yuhan refrains from entrusting or entrusting developing products and is building market competitiveness with its own products. The MFDS approved "Raboni-D," Yuhan's
- Policy
- An exception to the 1+3 Bill for IMD
- by Lee, Jeong-Hwan Jun 18, 2021 05:54am
- A letter from a pharmaceutical representative to the National Assembly affected the process of the National Assembly's Health and Welfare Committee's handling of generics, drug for data-based re-evaluation "1+3 bill." It was reflected in the revised schedule when a representative of company A sent a petition to 24 members of the National A
- Policy
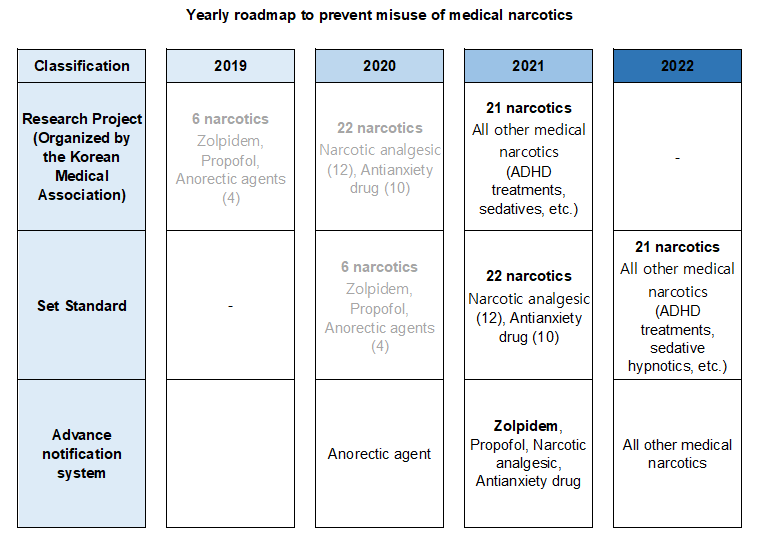
- 559 doctors warned for wrongful prescription of Zolpidem
- by Lee, Tak-Sun Jun 18, 2021 05:53am
- After analyzing the prescription information on the Narcotics Information Management System (NIMS), the Ministry of Food and Drug Safety announced its decision to issue written ‘warnings’ to 559 doctors that have continuously prescribed or used Zolpidem beyond the safe use standards to prevent abuse and promote proper use of the medical narcot
- Policy
- “The 1+3 bill” was passed by the Welfare Committee
- by Lee, Jeong-Hwan Jun 18, 2021 05:53am
- The 1+3 bill passed a plenary session of the National Assembly's Health and Welfare Committee on the morning of the 16th and will be reviewed by the legislation and judiciary committee. The resolution reflected the revision of the supplementary provision, which excludes the report from the MFDS within a month from the enforcement date of t
- Policy
- Moderna vaccine has been approved for lot release
- by Lee, Tak-Sun Jun 18, 2021 05:53am
- The MFDS said it approved lot release of 55,000 doses of Moderna COVID-19 vaccine on the 15th. The national lot release is a system in which country evaluates the results of the verification test and the review of the manufacturer's data before the vaccine is distributed on the market and checks the quality once more. The MFDS measured
- Policy
- AZ vaccine's side effects are reviewed
- by Lee, Tak-Sun Jun 17, 2021 08:02pm
- The MFDS has begun a review on the addition of side effects of capillary leakage syndrome of AstraZeneca's COVID-19 vaccine recommended by the European Medicines Agency (EMA). The MFDS announced on the 14th that it will take necessary measures such as distributing Dear Healthcare Professional Letter, sharing information recommended by EMA,
- Policy
- PBAC provides cost-effectiveness data to improve integrity
- by Lee, Hye-Kyung Jun 17, 2021 06:04am
- The Health Insurance Review and Assessment Service's Pharmaceutical Management Department was selected as 'best project' among 'self-promoted projects to improve integrity’ for its efforts in improving standard forms and expanding information disclosure systems. The department worked to improve integrity by clarifying and making transparent
- Policy
- Ruling/opposition parties fight over supply & COVID vaccine
- by Lee, Jeong-Hwan Jun 15, 2021 05:53am
- Political circles of the ruling and opposition parties have launched a special committee on the COVID-19 vaccine and TF respectively, and are checking each other over the status of vaccine supply and vaccination performance. The ruling party has been announcing the results of COVID-19 vaccine since the Korea-U.S. summit, while the main opp
- Policy
- President Moon talks with AZ CEO at G7 Summit
- by Kim, Jung-Ju Jun 15, 2021 05:53am
- South Korean President Moon Jae-in met with Pascal Soriot, CEO of AstraZeneca, to ask for the company’s continuous support in supplying COVID-19 vaccines to Korea in the second half of this year. AstraZeneca responded positively to the request and replied that it hopes to continue a long-standing relationship with SK Bioscience as one of it