- Policy
- There is progress in introducing additional COVID-19 vaccine
- by Lee, Jeong-Hwan Jan 14, 2021 06:15am
- Prime Minister Chung Sye-kyun announced that significant progress has been made in introducing additional vaccines from other platforms, including Pfizer, Moderna, AstraZeneca, Janssen, and others regarding the COVID-19 vaccine. Although the spread of COVID-19 has recently slowed, he has been wary of hasty measures to mitigate quarantine.
- Policy
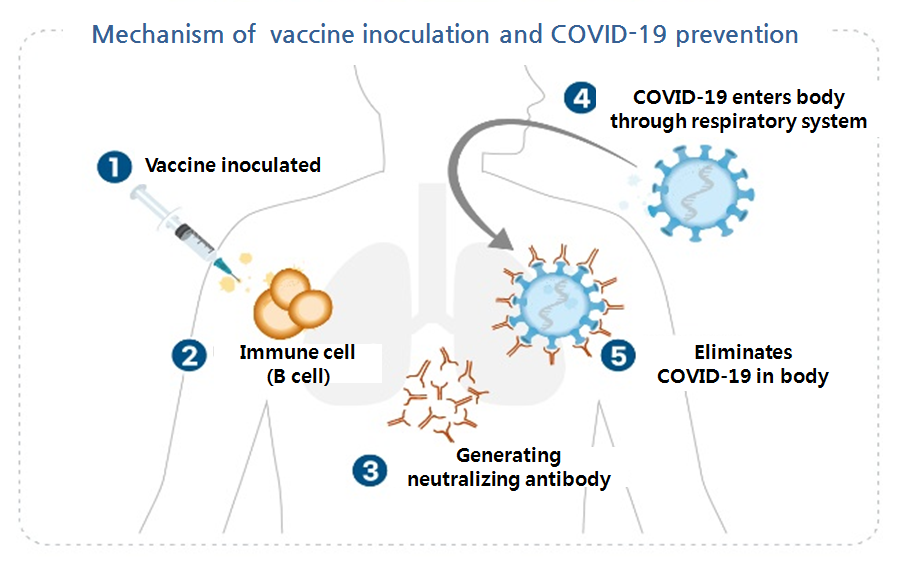
- All citizens will be vaccinated free of charge
- by Lee, Jeong-Hwan Jan 14, 2021 06:15am
- President Moon Jae-in promised free vaccination for all citizens according to the priority of the COVID-19 vaccine at the New Year's address on the 11th. It also announced a plan to strengthen vaccine sovereignty with the domestic COVID-19 vaccine, and to transparently disclose the entire process of licensing, including the safety and e
- Policy
- MFDS OK's removing “redundant” narcotics return approval
- by Lee, Jeong-Hwan Jan 13, 2021 06:12am
- The Ministry of Food and Drug Safety (MFDS) and the National Assembly Expert Committee Office approved of a bill to remove the MFDS narcotics pre-return approval procedure. The bill received the nod prior to the National Assembly Health and Welfare Committee’s review. When it is passed, the pharmacists’ convenience in returning the narcoti
- Policy
- 9 Atozet generic companies to join CKD via consignment
- by Lee, Tak-Sun Jan 12, 2021 06:22am
- Nine pharmaceutical companies, initially preparing to launch a generic version of a dyslipidemia treatment Atozet (atorvastatin plus ezetimibe), joined a group of manufacturers to produce Chong Kun Dang’s evidence-submitting drug as CMO. Instead of generics applying for the health authority approval from this month, the companies seem
- Policy
- Breaking down MOHW 2021 action plan for NHI Master Plan
- by Kim, Jung-Ju Jan 12, 2021 06:19am
- The drug reimbursement benefit would be improved throughout chronic disease treatment this year, centering hepatitis B and C virus treatment and antidiabetic combination drugs. The already listed drug reimbursement reevaluation would be enforced from the latter half of the year, when the subjects are decided in the first half of the year. Mo
- Policy
- 22 generics for Atozet by Chong Kun Dang were approved
- by Lee, Tak-Sun Jan 11, 2021 06:10am
- The products of 22 companies for hyperlipidemia (Ezetimibe/Atorvastatin) consigned by Chong Kun Dang were approved on the 8th. As a result, 20 generisc within the same ingredient were approved, and the next drug price application for the same drug product was reduced to the lowest price. This is because of the stepped drug price system tha
- Policy
- Plasma treatment was completed in Phase II clinical trial
- by Lee, Tak-Sun Jan 11, 2021 06:10am
- The government confirmed that the patient administration of the clinical II trial of a domestically developed blood system drug has been completed. As in the case of Celltrion's antibody treatment, the application for Conditional Marketing Authorization (CMA) is imminent. Kwon Jun-wook, the 2nd vice-president of the Central Disease Control
- Policy
- Adenovirus for AZ vaccine, Sinopharm uses inactivated virus
- by Lee, Tak-Sun Jan 11, 2021 06:10am
- Diverse types of COVID-19 vaccines are currently in development. Pfizer and Moderna’s vaccine candidates are based on RNA, when AstraZeneca’s is a virus vector and Korean-based SK Bioscience’ is a recombinant vaccine. These types of vaccine have their respective strengths and weaknesses. Some types have been commercialized already, but m
- Policy
- Boehringer's new obesity drug is conducting clinical trial
- by Lee, Tak-Sun Jan 8, 2021 06:19am
- Boehringer Ingelheim is conducting a clinical trial of a new obesity treatment in Korea. It is noteworthy whether it will exceed the sales of Saxenda (Liraglutide·NovoNordisk), which is currently leading in the Korean market. The MFDS approved the Phase II clinical trial protocol of BI 456906, a candidate for the new obesity drug of Boehr
- Policy
- We'll respond more aggressively through vaccines & tx
- by Kim, Jung-Ju Jan 8, 2021 06:19am
- President Moon Jae-in predicted that it will be able to respond more aggressively to COVID-19 as the commercialization and supply of vaccines and treatments will become reality next month. In particular, President Moon predicted that it would become a model country equipped with quarantine, vaccines, and treatments if commercialized, such