- Policy
- Boryung’s Pomalyst generic soon to be released
- by Lee, Tak-Sun Jan 14, 2025 05:57am
- Boryung's multiple myeloma drug Pomalikin Cap may soon be released in Korea’s market. The drug is the first generic version of Pomalyst Cap (pomalidomide, BMS). The risk-sharing agreement (RSA) applied to the original drug, Pomalyst Cap, ended this month, and the insurance price cap of the drug had been adjusted following the reimburseme
- Policy
- Will 'Lorviqua' secure a deal on drug price negotiations?
- by Lee, Tak-Sun Jan 13, 2025 05:53am
- The reimbursement expansion case for Lorviqua (lorlatinib, Pfizer), which was stalled during the drug price negotiation last year, has passed the Drug Reimbursement Evaluation Committee (DREC) of the Health Insurance Review and Assessment Service (HIRA) under a conditional term. It draws attention to whether it will succeed this round. Pfi
- Policy
- New criteria set for unstable supply and demand drugs
- by Lee, Tak-Sun Jan 13, 2025 05:53am
- The Health Insurance Review and Assessment Service has decided to clarify the criteria for evaluating adjustment applications for drugs with unstable supply and demand and strengthen preliminary management of high-priced anticancer drugs. HIRA announced so on the 9th while issuing a prenotification of the “Partial Amendments to the Eva
- Policy
- Chinese pharma targeting KOR, BeiGene gets greenlight
- by Lee, Hye-Kyung Jan 10, 2025 05:52am
- BeiGene Korea expands market dominance by conducting clinical trials to achieve competitiveness in the Korean market. Based on this year's Ministry of Food and Drug Safety (MFDS)'s clinical trial approval report, two out of ten approved clinical trial applications were BeiGene Korea's Phase 1 clinical trials. BeiGene has been strengthen
- Policy
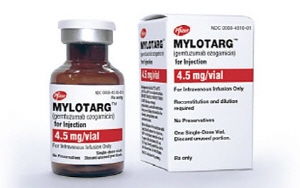
- AML treatment Mylotarg stalled at the DREC review
- by Lee, Tak-Sun Jan 9, 2025 05:56am
- The high price was found to be the reason Pfizer Korea's new drug Mylotarg (gemtuzumab ozogamicin), a treatment for acute myeloid leukemia (AML), did not pass the Health Insurance Review and Assessment Service (HIRA) stage. HIRA acknowledged the drug's improvement in clinical utility but did not approve its cost-effectiveness. According
- Policy
- Dong-A ST first to pay the raised new drug approval fee?
- by Lee, Hye-Kyung Jan 7, 2025 06:05am
- With the new drug approval fee raised to KRW 410 million on Jan. 1 this year, the industry is eyeing whether Dong-A ST will become the first to apply for marketing authorization and pay the raised new drug approval fee. According to industry sources on the 7th, Dong-A ST applied for a prior consultation before applying for marketing authoriza
- Policy
- Zolgensma shows 94% effect during post-evaluations
- by Lee, Tak-Sun Jan 6, 2025 05:56am
- The spinal muscular atrophy (SMA) treatment Zolgensma (onasemnogene abeparvovec) showed promising results suited to its high price. The use of the once-daily, one-shot treatment, which has an insurance cap set at KRW 2, requires a post-marketing evaluation to measure its cost-effectiveness. According to industry sources on the 5th, Z
- Policy
- MFDS’s 2nd GIFT drug Nefecon applies for reimbursement
- by Lee, Tak-Sun Jan 6, 2025 05:56am
- Nefecon (micronized budesonide), the only drug approved by the U.S. FDA to treat lgA (immunoglobulin A) nephropathy, has applied for domestic health insurance reimbursement coverage in Korea. The drug was approved in November last year as its 2nd GIFT (Global Innovative Drug Fast Track) drug, MFDS’s fast track program. According to in
- Policy
- 'Pivlaz Inj' makes another attempt to be reimb in KOR
- by Lee, Tak-Sun Jan 3, 2025 06:27am
- 'Pivlaz Inj,' which is supplied·distributed in South Korea by Handok, has been submitted for another attempt at reimbursement. The marketing authorization for Pivlaz was approved in South Korea in December 2023. Then, the company applied for reimbursement but voluntarily withdrew the reimbursement application in June 2024. According t
- Policy
- New Year's drug pricing policy highlights
- by Lee, Tak-Sun Jan 3, 2025 06:27am
- The new drug pricing system in 2025 is expected to be volatile due to Korea’s political turmoil. Whether the external reference pricing reevaluations and re-evaluation of the listed PE exemption drugs, which were reviewed or discussed last year, will be carried out this year is also the focus of attention. However, as most of the detai