- Policy
- Ongentys and Rekovelle soon to receive healthcare benefit
- by Kim, Jung-Ju Aug 11, 2020 06:02am
- The South Korean health authority is readying for healthcare reimbursement listing on SK Chemical’s new Parkinson’s disease treatment Ongentys (opicapone) capsule and Ferring Korea’s assisted reproduction techniques (ART) add-on Rekovelle (follitropin delta) prefilled pen as they have reached an agreement on their negotiations. During
- Policy
- Benefits for combination therapy with DM can be expanded
- by Lee, Tak-Sun Aug 10, 2020 06:01am
- Combination therapy between diabetes treatment drugs that are not currently approved for efficacy and effectiveness can also receive benefits. As the MFDS simplifies the method of describing the efficacy and effect of type II DM combination therapy, the insurance authorities can apply benefits to the existing off-label combination therapy (us
- Policy
- Pricing reduction-evading drug relisting to be rejected
- by Kim, Jung-Ju Aug 10, 2020 06:00am
- Now a new regulation would stop companies from attempting to technically relist a drug to avoid pricing reduction. Following the administrative procedure, the newly revised regulation would be enforced from September at earliest. The Regulatory Reform Committee categorized the Ministry of Health and Welfare (MOHW)-revised ‘Regulation on
- Policy
- Pre-approval of benefits for Spinraza were approved
- by Lee, Hye-Kyung Aug 10, 2020 06:00am
- 5 out of 6 cases for pre-approval of benefits for Spinal Muscular Atrophy (SMA) treatment 'Spinraza (Nusinersen)' last month were approved. After approval of Spinraza benefits, a monitoring report must be submitted every four months before administration of maintenance doses, and all 24 cases of administration monitoring have been approv
- Policy
- Kcab, compared clinical trial with Nexium
- by Lee, Tak-Sun Aug 7, 2020 06:27am
- HK inno.N's new domestic drug ‘Kcab’ is specifically compared with the reference drug, Nexium. The company said it will explore new indications through the trial. The MFDS approved IND of Phase I clinical trial to explore the pharmacokinetics, Pharmacodynamic properties and safety of Tegoprazan twice a day dosing regimen in healthy subj
- Policy
- SNUH participates in COVID-19 trials using Rebif
- by Lee, Tak-Sun Aug 7, 2020 06:27am
- Seoul National University Hospital participates in a multinational clinical trial using 'Rebif' (Interferon Beta-1A, Merck), which has recently been cited as a candidate for COVID-19 treatment. Rebif is a pre-filled injection that is also approved in Korea. It is used for multiple sclerosis, and has recently been mentioned as a candidate f
- Policy
- Generics was applied in 7 years after Pelrubi’s PMS ended
- by Lee, Tak-Sun Aug 7, 2020 06:26am
- The application for generic for Pelubi was received 7 years after the PMS of the original drug was terminated. There have been reasons for not being able to apply because of patents, but it is an analysis that Pelubi's popularity in the market lately has influenced the generic development of pharmaceutical companies. According to the MFDS
- Policy
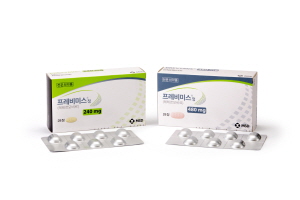
- Pricing settled Prevymis to get listed in Aug. at earliest
- by Kim, Jung-Ju Aug 7, 2020 06:25am
- The MSD Korea’s new drug Prevymis (letermovir) settled on a pricing negotiation with the South Korean health authority and successfully passed the biggest hurdle of the path to healthcare benefit. The novel drug helps to prevent cytomegalovirus (CMV) infection in patients who have received an allogeneic hematopoietic stem cell transplan
- Policy
- Zytiga, Mavyret and 161 items monitored for PVA negotiation
- by Lee, Hye-Kyung Aug 5, 2020 06:24am
- The South Korean health authority added Korea Otsuka Pharmaceutical’s Iclusig tablet (ponatinib hydrochloride hydrate), Jassen Korea’s Imbruvica capsule (ibrutinib), Zytiga tablet (abiraterone acetate) and Darzalex (daratumumab) as subject for the third quarter price-volume agreement (PVA) negotiation and monitoring. Merck’s Erbitux i
- Policy
- COVID-19 vaccine release time can be judged in phase III
- by Lee, Hye-Kyung Aug 4, 2020 05:54am
- With the time to release the COVID-19 vaccine, a domestic expert said that it is not yet in the stage of discussion. Mook Hyun-sang, the head of Korea Drug Development Fund (KDDF) announced the results at the future open discussion with the theme of 'COVID-19 vaccine, global development trend and securing strategy' co-sponsored by the MO