- Policy
- Chong Kun Dang’s capsuled low-dose Xarelto generic approved
- by Lee, Tak-Sun Apr 14, 2020 06:11am
- Chong Kun Dang’s generic version of Bayer’s anticoagulant Xarelto (rivaroxaban) tablet was approved as a first capsule form in Korea. The 2.5 mg low-dose capsule would be able to evade Hanmi Pharmaceutical and SK Chemical’s preferential sales rights on their generic products with the change in administration form. On Apr. 9, Minist
- Policy
- Controversy concludes about COVID-19 & Safety of HTN drugs
- by Lee, in-bok Apr 13, 2020 06:13am
- It is expected that clinical studies will begin in Korea to determine the association between COVID-19 and angiotensin converting enzyme (ACE) -based hypertensive drugs. It is a willingness to uncover whether it can be a threat to domestic hypertensive patients in terms of controversial issues worldwide. It was confirmed that the publ
- Policy
- President Moon “Expediting COVID-19 treatment approval”
- by Lee, Jeong-Hwan Apr 13, 2020 06:13am
- Amid COVID-19 pandemic, President Moon Jae-in of South Korea has expressed his expectation for Korean-made treatment and vaccine to save the humanity. President Moon personally emphasized the government support for the COVID-19 treatment vaccine through R&D investment and expedited approval process. At a meeting convened on Apr. 9 fo
- Policy
- 161 items, subject to monitoring for Price-Volume agreement
- by Lee, Hye-Kyung Apr 13, 2020 06:12am
- AstraZeneca Korea's Tagrisso 40mg/80 mg (Osimatinib Mesylate) were added to the monitoring target for the price-volume agreement negotiation system in the second quarter of this year. Celgine's Pomalyst (Pomalidomide) 1·2·3·4 mg, Roche Korea's Tecentriq (Atezolizumab), and Jansen Korea's Sylvant (Siltuximab) 100·400 mg are also subject
- Policy
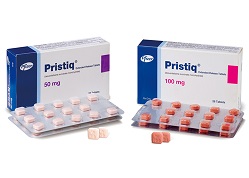
- Salt-altering drugs for Pristiq ER got generic exclusivity
- by Lee, Tak-Sun Apr 13, 2020 06:12am
- Pfizer's first generic drug for anti-depressant drug Pristiq ER (Desvenlafaxine) was also approved as generic exclusivity. Accordingly, four generics of Whan In, Myungin, Nexpharm Korea, Hallim, will be able to monopolize in the same ingredient formulation market until January 8, next year. On the 8th, the MFDS approved Whan In’s Defax
- Policy
- P-CABs, compete for indication expansion
- by Lee, Tak-Sun Apr 10, 2020 06:29am
- Potassium-Competitive Acid Blocker (P-CAB) drugs, which are called next-generation gastric acid inhibitors, are spurring to expand indications. Currently, the only drug released in Korea is K-Cab by HK inno.N (Tegoprazan). As Vocinti (Vonoprazan by Takeda) is getting permission and is planning to release, Daewoong Pharmaceutical's Fexupra
- Policy
- The first generics for Pristiq ER were approved
- by Lee, Tak-Sun Apr 10, 2020 06:28am
- The generic drugs that avoided the patent of Pristiq ER obtained the item permission. They are expected to be competitive as first generics in the market. The Ministry of Food and Drug Safety approved the approval of four items of Desvenlafaxine, generic for Pristiq ER on the 7th. The generics are Defaxine SR 50·100mg by Whan In, Desve
- Policy
- AbbVie does not enforce Kaletra’s patent right
- by Lee, Tak-Sun Apr 9, 2020 09:43pm
- AbbVie’s Kaletra (Lopinavir/Ritonavir), which is used in the clinical field as a treatment for COVID-19, has abandoned patent rights, but there is no news of generic development in Korea This is because the market size of AIDS treatment (HIV-1), which is the main indication of Kaletra, is not large in Korea and is already occupied by othe
- Policy
- Can generic listing negotiation prevent reckless listing?
- by Kim, Jung-Ju Apr 9, 2020 06:26am
- Although some pharmaceutical companies are voicing their concerns over the generic reimbursement negotiation, to be enforced from the second half of the year, some are actually welcoming the new system with an anticipation of positive benefits. Along with the revised drug pricing system coming in effect around the same time, the generic n
- Policy
- GV1001 developed as a demetia drug, approved for COVID-19
- by Lee, Tak-Sun Apr 8, 2020 06:21am
- The Ministry of Food and Drug Safety approved a new drug candidate 'GV1001' by GemVax, which is being developed as a treatment for dementia, for the purpose of treating COVID-19 patients. The request for approval for the use of treatment was made by Kyungpook National University Chilgok Hospital. The MFDS approved the use of GV1001 (Ter