- Policy
- Will Rovelito by Handok & Sanofi succeed?
- by Lee, Tak-Sun Apr 7, 2020 06:44am
- Handok & Sanofi-aventis Korea are jointly developing a combination of Irbesartan & Amlodipine, which is antihypertensive drug and are expecting synergy effect between the two companies. Previously, Sanofi-aventis Korea co-developed Rovelito (Irbesartan-Atorvastatin calcium trihydrate), a new combination drug for hypertension/ hyperlipidem
- Policy
- Samjin’s generic for Forxiga was first approved
- by Lee, Tak-Sun Apr 7, 2020 06:39am
- Samjin's SGLT-2 diabetes treatment drug 'Forxiga' was first approved. Unlike Forxiga, it is a product without solvates. Through this, it is expected to avoid the solvate patent and weigh the release around April 2023 when the material patent ends. On the 2nd, the Ministry of Food and Drug Safety approved Samjin Pharmaceutical's Dapazin
- Policy
- FDA requests removal of all Ranitidines from the market
- by Lee, Tak-Sun Apr 6, 2020 06:27am
- As the Food and Drug Administration (FDA) decides to withdraw all products containing Ranitidine on the 1st, domestic pharmaceutical companies seeking to resume sales are embarrassed Until recently, some Ranitidine products were on sale in the US market, so it was expected that if the stability data were submitted in Korea, it would be po
- Policy
- The first generic for Afinitor & Betmiga will be released
- by Lee, Tak-Sun Apr 6, 2020 06:26am
- Guangdong Pharmaceutical is licensed for the first generic version of the breast cancer treatment drug Afinitor (Everolimus, Novartis) and is expected to launch this year. In addition, Hanmi Pharm and Chong Kun Dang also received the first generic approval of the overactive bladder treatment drug 'Betmiga' (Mirabegron, Astellas). These pro
- Policy
- Will Hanmi and Chong Kun Dang's generic exclusivity effect?
- by Lee, Tak-Sun Apr 3, 2020 06:35am
- It is noteworthy whether Hanmi Pharm or Chong Kun Dang among the domestic pharmaceutical companies will be able to achieve the performance in the market while acquiring generic exclusivity, which is the most outstanding pharmaceutical company. In particular, attention has been focused on Hanmi and Chong Kun Dang in that there are no succes
- Policy
- Rumor: Chief Yang as new Pharmaceutical Benefits Director
- by Kim, Jung-Ju Apr 3, 2020 06:33am
- Apparently, a rumor is circulating about the new director of Pharmaceutical Benefits Division at Ministry of Health and Welfare (MOHW), who would lead the Moon Jae-in Care-initiated drug pricing system revision. According to pharmaceutical industry sources on Mar. 31, Chief Yang Yoon Seok of Smart Healthcare Regulation Revision Planning
- Policy
- New drug reimbursement review costs KRW 39 million
- by Lee, Hye-Kyung Apr 2, 2020 06:26am
- Experts recommend the Korean government should review implementing service fee system for reimbursement review to enhance speed and efficiency of pharmaceutical reimbursement listing procedure, and to provide quality service through sufficient financial and human resources. While the Health Insurance Review and Assessment Service (HIRA) does
- Policy
- Marketing approval for major Ranitidines have been renewed
- by Lee, Tak-Sun Apr 2, 2020 06:26am
- The approval of Ranitidine formulations, such as Albis, Curan, and Zantac, which have been banned from the end of September last year due to the detection of carcinogenic substances NDMA, has been renewed. Accordingly, permits will remain in effect until March 31, 2025. It is noted whether sales can be resumed during this period. According
- Policy
- NHIS to act on growing number of administrative litigations
- by Lee, Hye-Kyung Apr 2, 2020 06:26am
- National Health Insurance Service (NHIS) is setting down administrative litigation response strategy to handle pharmaceutical companies’ taking legal action against drug pricing reduction. NHIS has recently started a cosigned research on ‘Drug Pricing Reduction Related Litigation Case Study and Response Plan.’ The research would be
- Policy
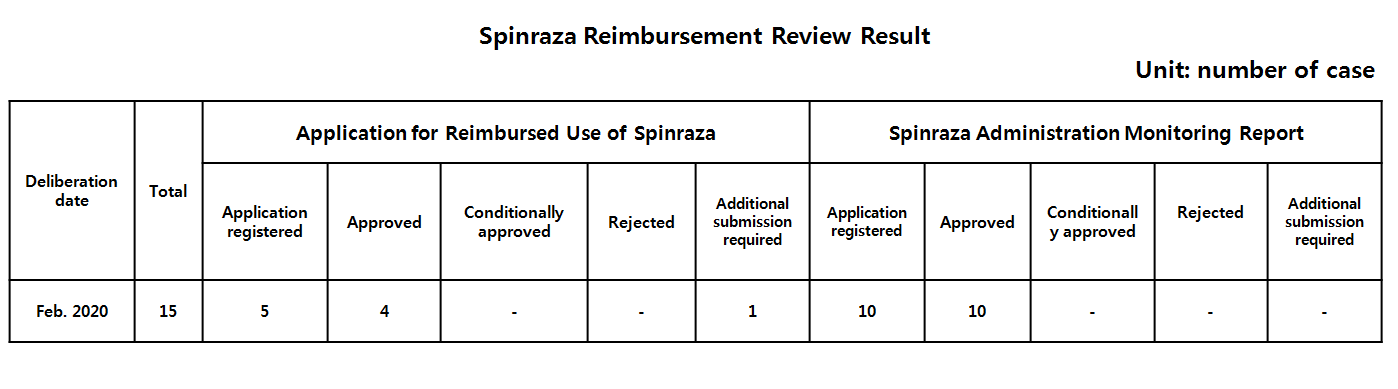
- HIRA clears 4 out of 5 Spinraza reimbursement pre-approvals
- by Lee, Hye-Kyung Apr 2, 2020 06:26am
- Korean health authority has passed four out of five preliminary applications submitted last month to treat spinal muscular atropy (SMA) with reimbursed use of Spinraza. Even the one denied case would likely to be cleared, if the applicant submits additional evidential data of patient’s onset symptoms of SMA. In every four months, Sp