- Policy
- Novartis cuts price of Cosentyx after PVA negotiations
- by Lee, Tak-Sun Nov 7, 2024 05:46am
- The price of Novartis’s Cosentyx(secukinumab) is set to be reduced due to its increased use. This is because the National Health Insurance Service recently reached an agreement during Price-Volume Agreement negotiations with the company for Cosentyx. According to industry sources on the 6th, the insurance price ceiling of Cosentyx wa
- Policy
- 'Eutropin·Biktarvy' price drop amid sales hike
- by Lee, Tak-Sun Nov 6, 2024 05:52am
- Due to the increased volume of usage, drug pricing for 'Eutropin Inj (somatropin, LG Chem)' and 'Biktarvy (Bictegravir/Emtricitabine/Tenofovir Alafenamide, Gilead Science),' which recently showed a sales hike, is expected to be reduced. Sources said on November 5th that the Health Insurance Review and Assessment Service (HIRA) recently
- Policy
- How will the new drug review process change with fee hike?
- by Lee, Hye-Kyung Nov 6, 2024 05:52am
- The Ministry of Food and Drug Safety (MFDS) announced plans to raise the fee for new drug approvals to KRW 410 million from January 1 next year. The agency has prepared the approval and review process to implement the plan and has begun collecting industry opinion. According to MFDS’s press corp coverage, the MFDS recently delivered a revise
- Policy
- Reimb standards that prevent abuse of HA Eye Drops imminent
- by Lee, Tak-Sun Nov 5, 2024 05:45am
- Health authorities are expected to establish reimbursement standards to prevent the misuse of hyaluronic acid eye drops after failing to reach a conclusion during last year's reimbursement adequacy evaluations. It is reported that the Ministry of Health and Welfare is preparing an administrative notice to announce the reimbursement standar
- Policy
- Anticipating reimb for DPP4·SGLT2 combination drugs
- by Lee, Jeong-Hwan Nov 1, 2024 05:51am
- The government has announced a plan to address the necessity of the National Health Insurance reimbursement·expansion for two-drug combination drugs containing DPP-4 inhibitors and SGLT-2 inhibitors for the treatment of type 2 diabetes. In South Korea, reimbursement for combination drugs containing DPP-4 inhibitors and SGLT-2 inhibitors
- Policy
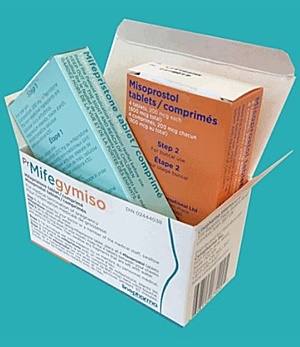
- Hyundai withdraws application for Mifegymiso approval again
- by Lee, Hye-Kyung Nov 1, 2024 05:50am
- Hyundai Pharm has reportedly voluntarily withdrawn the application it had filed for the marketing authorization of Mifegymiso (mifepristone and misoprostol), which the company had applied for approval as the first abortion pill in Korea. Hyundai Pharm has requested approval for the abortion drug twice in the past, first in 2021 and then
- Policy
- Seretide withdraws from Korea due to poor performance
- by Lee, Tak-Sun Oct 31, 2024 05:55am
- GSK's asthma inhaler 'Seretide' is withdrawing from the domestic market. Once the market leader in the market, it seems that the company is reorganizing the product line as its performance has recently declined due to the entry of new products. According to industry sources on the 30th, GSK recently sent letters to long-term care organiza
- Policy
- Gvnt agrees on the need for dispensing substitute drugs
- by Lee, Jeong-Hwan Oct 30, 2024 05:54am
- In this year's NA Audit, the Minister of Health and Welfare Kyoo-Hong Cho said that he would prioritize the ‘dispensing of substitute drugs’ as a solution to the problem of unstable supply of medicines such as cold medicines that are often out of stock. Two bills related to alternative dispensing were submitted to the National Assembly
- Policy
- Low reimb approval rate hinders Soliris’s use for aHUS
- by Lee, Tak-Sun Oct 29, 2024 05:49am
- The industry’s eyes are on whether the preliminary reimbursement review process for Soliris, a treatment for the rare disease aHUS (atypical hemolytic uremic syndrome), will be eased in Korea. Until now, patients wishing to use Soliris for aHUS with reimbursement had to pass a preliminary review process. However, the problem is its low
- Policy
- COVID-19 treatments 'Paxlovid·Veklury' reimb begins today
- by Lee, Jeong-Hwan Oct 28, 2024 05:54am
- Beginning today (October 25th), the National Health Insurance is applied to COVID-19 treatments, Paxlovid Tab (Pfizer Korea) and Veklury Inj (Gilead Sciences Korea). The patient copay will be maintained at the current cost of about KRW 50,000: KRW 47,090 for a single package of Paxlovid Tab (30 tablets) and KRW 49,920 (6 bottles) for Vekl