- Policy
- Takeda's drug pricing negotiations see different results
- by Lee, Tak-Sun Sep 25, 2024 05:48am
- The drug pricing negotiations for ‘Ceprotin (Protein C Concentrate (Human))’ a new drug for severe congenital protein C deficiency supplied by Takeda Pharmaceuticals Korea, fell through. On the other hand, the company reached an agreement and completed negotiations to expand the reimbursement for the ovarian cancer treatment Zejula Cap.
- Policy
- Pharmbio releases first generic Revolade with reimb in OCT
- by Lee, Tak-Sun Sep 24, 2024 05:46am
- The first generic version of the immune thrombocytopenia treatment ‘Revolade&160;(eltrombopag&160;olamine)’ will be released into the market in October. Pharmbio Korea’s Revolade generic has succeeded in receiving reimbursement listing 1 year after its approval in Korea. The company had been delaying its launch due to the burden of p
- Policy
- AZ's bispecific ab 'Rilvegostomig' vs Keytruda trial
- by Lee, Hye-Kyung Sep 20, 2024 05:51am
- A Phase 3 trial comparing a combination therapy containing 'Rilvegostomig (Trial name: AZD2936),' AstraZeneca's bispecific antibody gathering attention as the next-generation medicine, to 'Keytruda (pembrolizumab),' a type of immunotherapy drug used to treat cancer, will be conducted in South Korea. The Ministry of Food and Drug Safety (M
- Policy
- Cushing’s syndrome drug Isturisa is approved in Korea
- by Lee, Hye-Kyung Sep 20, 2024 05:51am
- Various doses of ‘Isturisa’ (osilodrostat), a treatment for Cushing's syndrome, are expected to be approved in Korea soon. According to industry sources on the 19th, the Ministry of Food and Drug Safety concluded the safety and efficacy review of Recordati Korea's Isturisa Film Coated Tab 1mg earlier this month and recently completed th
- Policy
- MFDS approves Hemgenix for Hemophilia B in Korea
- by Lee, Hye-Kyung Sep 19, 2024 05:48am
- The Ministry of Food and Drug Safety (MFDS) announced on the 13th that it has approved the orphan drug ‘Hemgenix Inj (etranacogene dezaparvovec)’ imported by CSL Behring Korea. Hemgenix is used to treat moderate-to-severe hemophilia B (congenital blood clotting factor IX deficiency) in adults without a history of Factor IX inhibitors. T
- Policy
- Moderna’s COVID-19 vaccine is approved in Korea
- by Lee, Hye-Kyung Sep 13, 2024 05:50am
- A new COVID-19 variant vaccine, which is the only mRNA-based COVID-19 vaccine that will be manufactured domestically (by Samsung Biologics) has been approved in Korea. The Ministry of Food and Drug Safety (MFDS, Minister: Yu-Kyoung&160;Oh) announced on the 11th that it has authorized the manufacturing and sale of ‘Spikevax JN.1’ that M
- Policy
- Price of Pristiq original and IMDs cut with generic release
- by Lee, Tak-Sun Sep 11, 2024 05:54am
- The release of generic versions of Pfizer's antidepressant ‘Pristiq ER Tab,’ has hit not only the sales of the original drug but also the salt-modified version. Korea Pharma launched the first generic version of Pristiq in Korea last month, and the release has also affected the salt-modified drug that was released 4 years ago. Accordi
- Policy
- MFDS announces EUA application of new COVID-19 vaccine
- by Lee, Hye-Kyung Sep 9, 2024 05:49am
- An emergency use authorization process is in progress for new COVID-19 vaccines so that the disease control authorities can inoculate using the vaccines in October. On the 6th, the Ministry of Food and Drug Safety announced an application for emergency use approval of the ‘JN.1 variant-response recombinant protein vaccine for the prevent
- Policy
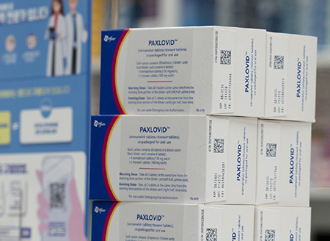
- COVID-19 drug Paxlovid’s price likely to be based on Japan
- by Lee, Tak-Sun Sep 6, 2024 05:48am
- The insurance ceiling price of Pfizer's COVID-19 drug Paxlovid is likely to be set at around KRW 900,000 in Korea. This is similar to its price in Japan. Japan is said to have the lowest price for Paxlovid among the A8 countries. According to industry sources on the 4th, Paxlovid passed the Drug Reimbursement Evaluation Committee (DREC
- Policy
- ‘Policy support enabled the prompt approval of Envlo’
- by Lee, Hye-Kyung Sep 4, 2024 05:49am
- The backstory of Daewoong Pharmaceutical's Envlo 0.3mg (enavogliflozin), which is a representative success case of the MFDS’s expedited review process, was introduced recently. The government’s policy support for the commercialization of innovative products was what enabled Envlo’s clinical trial to approval in just 5 years. In general