- Policy
- ‘Exclude GER and CAN or cut price by 50% less'
- by Lee, Tak-Sun Jul 15, 2024 05:48am
- As the government is pushing for external reference pricing reevaluations, the pharmaceutical industry has proposed two alternatives: excluding Germany and Canada from the A8 countries used for comparison or reducing the drug price cut rate by 50%. The government is reportedly reviewing the proposed options. The industry has taken a ha
- Policy
- Roche Korea speeds up reimb of Ocrevus in KOR
- by Lee, Tak-Sun Jul 12, 2024 05:48am
- Roche Korea is making active moves to receive reimbursement for its multiple sclerosis drug Ocrevus, which was approved in May this year. Roche, which applied for reimbursement immediately after the approval, has set out to persuade the Health Insurance Review and Assessment Service to expedite the application process. According to in
- Policy
- Reassessing the plan for comparing foreign drug prices
- by Lee, Tak-Sun Jul 12, 2024 05:48am
- The government will likely reassess foreign drug price comparison by evaluating the therapeutic category with the highest number of products. Consequently, gastrointestinal agents, high blood pressure drugs, and antibiotics will be assessed in the first year. There are over 2000 products in these three therapeutic categories. Furthermore
- Policy
- ‘Bioequivalence tests rather intensified price cuts'
- by Lee, Tak-Sun Jul 12, 2024 05:47am
- The pharmaceutical industry is calling for improvements as their drugs that have completed bioequivalence tests per the government’s insurance price ceiling reevaluations may face larger price cuts under ‘Type C’ of the Price Volume Agreement negotiations this year. Generic drugs that have completed bioequivalence tests during the i
- Policy
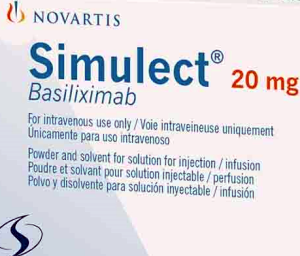
- Simulect in supply crisis with no alternative
- by Lee, Tak-Sun Jul 11, 2024 05:47am
- Simulect Inj (basiliximab, Novartis Korea), which is used as an induction therapy to prevent immune rejection in organ transplant patients, is expected to run out of supply in Korea due to issues in securing the supply the drug’s active pharmaceutical ingredient. In particular, there is no alternative drug for liver transplantation, in
- Policy
- Debate within the Health and Welfare Committee members
- by Lee, Jeong-Hwan Jul 10, 2024 05:48am
- Members of the Democratic Party of Korea and the ruling party who are also committee members of the Health and Welfare Committee of the National Assembly show significant differences in viewpoints regarding President Yoon Suk Yeol’s policy of expanding medical school quotas, and the current situation surrounding the conflict between the med
- Policy
- Reimbursement application submitted for 'Adempas tab'
- by Lee, Tak-Sun Jul 10, 2024 05:48am
- Bayer’s 'Adempas tab,' a treatment for chronic thromboembolic pulmonary hypertension (CTEPH), is being considered for reimbursement listing 10 years after its approval as a novel drug. According to industry sources on July 2nd, a reimbursement decision application for Adempas was recently submitted to the Health Insurance Review and Ass
- Policy
- 1st bispecific antibody Epkinly approved with a condition
- by Lee, Hye-Kyung Jul 9, 2024 05:51am
- 'Epkinly (epcoritamab),' the first T-cell&8211;engaging bispecific&160;antibody, was found to have been approved under the condition that the company submits Phase III trial data. In Korea, the approval was granted on March 20 based on Phase II clinical trial data and a Phase III trial protocol, but at the time, a condition was added th
- Policy
- BMS’ Camzyos and Handok’s Empaveli pass the DREC review
- by Lee, Tak-Sun Jul 9, 2024 05:51am
- Bristol Myers Squibb Korea’s 'Camzyos Cap,' a treatment for symptomatic obstructive hypertrophic cardiomyopathy (oHCM), has passed the Drug Reimbursement Evaluation Committee (DREC) review after reconsideration. Handok’s 'Empaveli Inj,' a treatment for paroxysmal nocturnal hemoglobinuria (PNH), also obtained approval for reimbursement and m
- Policy
- Reassessing the comparison of foreign drug pricing is 'idle'
- by Lee, Tak-Sun Jul 8, 2024 05:46am
- The 10th public-private meeting has been held to reassess the comparative methods of foreign drug pricing. However, they exchanged different opinions without reaching a concrete decision. Pharmaceutical industry has particularly opposed to the use of drug pricing references from Germany and Canada. It remains to be seen how the two part