- Policy
- 10th external reference pricing reeval meeting is held
- by Lee, Tak-Sun Jul 5, 2024 05:48am
- The 10th meeting for the external reference pricing reevaluations is scheduled to be held this Friday (July 5). The meeting, which was scheduled to be held on June 27th, has been postponed to this week. The meeting between the government and the pharmaceutical industry, which has been held since November last year, is being held for th
- Policy
- Daewoong·Ildong late entries to the dapagliflozin market
- by Lee, Tak-Sun Jul 4, 2024 05:52am
- Daewoong Pharmaceutical and Ildong Pharmaceutical have made late entries in the market for diabetes treatment containing 'dapagliflozin.' Although the door to the biosimilar market for dapagliflozin opened in April last year, these two companies did not join the market immediately. It was presumed because of the contract binding with Astra
- Policy
- GSK will discontinue supply of Infanrix IPV
- by Lee, Tak-Sun Jul 2, 2024 05:48am
- GlaxoSmithKline has decided to discontinue supply of its ‘Infanrix IPV’ vaccine, which protects against diphtheria, tetanus, pertussis, and polio in children, in South Korea. Infanrix IPV and Sanofi Pasteur's ‘Tetraxim’ are the only DTaP-IPV vaccines used in the National Immunization Program (NIP), raising concerns about the shortage o
- Policy
- Expenses for the major four severe diseases tops KRW 7T
- by Lee, Tak-Sun Jul 1, 2024 05:47am
- Pharmaceutical expenses for the four major severe diseases exceeded KRW 7 trillion last year. The four major severe diseases refer to cancer diseases, cerebrovascular disease, and rare·severe incurable diseases. Pharmaceutical expenses for this category have risen due to high-cost pharamceuticals. Consequently, an analysis suggests that m
- Policy
- Gov't releases generic drug price comparison study results
- by Lee, Tak-Sun Jun 27, 2024 05:47am
- The government's decision to fully disclose the 'Study on Measures to Improve the Generic Drug Pricing System,' which ended in April, ahead of the final meeting for the external reference pricing reevaluations, is being analyzed as a sign that the authorities intend to push ahead its plan to cut generic drug price through reevaluations.
- Policy
- Some Akynzeo products recalled due to insufficient API
- by Lee, Hye-Kyung Jun 27, 2024 05:47am
- The Ministry of Food and Drug Safety (MFDS) recalled some lot numbers of HK Inno.N's antiemetic ‘Akynzeo Cap’ after confirming the possibility that the drug may not contain enough active&160;pharmaceutical ingredient (API). On the 21st, the MFDS issued a recall order for Akynzeo batch number '43000563 [2026-11-30].’ The reason fo
- Policy
- ‘Hypertension·hyperlipidemia generics are pricier in KOR'
- by Lee, Jeong-Hwan Jun 26, 2024 05:46am
- According to a government study, generic drugs for some indications, such as those for the gastrointestinal system, hypertension, and hyperlipidemia, are more expensive in Korea than in major overseas countries other than the United States, such as the United Kingdom, Switzerland, and Japan. As of 2022, Korean generic hyperlipidemia drugs we
- Policy
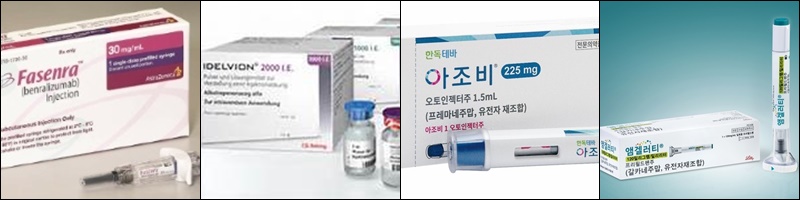
- Fasenra·Idelvion, Ajovy·Emgality receive reimb in KOR
- by Lee, Jeong-Hwan Jun 25, 2024 05:47am
- AstraZeneca's severe eosinophilic asthma treatment Fasenra (benralizumab) and CSL Behring's hemophilia B drug Idelvion (albutrepenonacog alfa) will be reimbursed by the national health insurance starting on the 1st of next month. Also, the anti-malignant tumor agent rituximab (original brand name: MabThera) and the migraine drug Ajovy (f
- Policy
- LG Chem and Samsung Bioepis's biosimilars are reimbursed
- by Lee, Tak-Sun Jun 25, 2024 05:46am
- LG Chem and Samsung Bioepis’ biosimilar products will be listed for reimbursement in July. LG Chem is launching a biosimilar of the autoimmune disease treatment Humira, and Samsung Bioepis is launching a biosimilar of Stelara. Both are aiming to list at the lowest price to compete with their respective original products. According to i
- Policy
- Combinatory drugs with linagliptin+dapagliflozin
- by Lee, Tak-Sun Jun 25, 2024 05:46am
- Following the patent expiration of DPP-4 inhibitor Trajenta (linagliptin), combinatory drugs containing linagliptin have been released. Next month, a combinatory drug containing linagliptin combined with SGLT-2 inhibitory dapagliflozin will enter the market. It is a combinatory drug with a new combination being introduced in South Korea. Acco