- Policy
- Erwinase price increases… Imfinzi renews RSA
- by Lee, Tak-Sun May 20, 2024 05:41am
- The price of Erwinase Inj (L-asparaginase), used to treat patients with acute lymphoblastic leukemia (ALL), is expected to increase. Additionally, Imfinzi, an immunotherapy drug for cancer, also succeeded in renegotiating risk-sharing agreements (RSA). Rhopressa, a treatment for open-angle glaucoma and intraocular pressure, has entered n
- Policy
- Daewoong develops 'Olumiant' generic…starts clinical trials
- by Lee, Hye-Kyung May 17, 2024 05:47am
- Daewoong Pharmaceutical has started developing product to compete against Lily Korea’s JAK inhibitor 'Olumiant (baricitinib).' Olumiant was approved by the Ministry of Food and Drug Safety (MFDS) in December 2017. It is used for treating adult patients with moderate-to-severe rheumatoid arthritis who do not respond well to disease-modi
- Policy
- Multiple sclerosis drug Ocrevusis approved in Korea
- by Lee, Hye-Kyung May 14, 2024 05:48am
- The Ministry of Food and Drug Safety (Minister: Yu-Kyung Oh) announced on the 13th that it has approved Roche Korea’s orphan drug Ocrevus (ocrelizumab) for multiple sclerosis (MS) in Korea. Multiple sclerosis is a chronic condition that develops in the central nervous system, which consists of the brain, spinal cord, and optic nerves and
- Policy
- BIO KOREA 2024 concludes a success
- by Lee, Hye-Kyung May 14, 2024 05:48am
- BIO KOREA 2024, cohosted by the Korea Health Industry Development Institute (President: Soon-do Cha) and the Provincial Administration of Chungcheongbuk-do (Governer: Young-hwan Kim), concluded successfully on the 10th. BIO KOREA 2024, which celebrates its 19th anniversary this year, was held for 3 days at COEX in Seoul under the theme of 'T
- Policy
- New dementia drug Leqembi’s approval imminent in Korea
- by Lee, Hye-Kyung May 14, 2024 05:48am
- The marketing authorization for Leqembi (lecanemab), the first drug to slow the progression of Alzheimer's disease, is imminent in Korea. According to industry sources on the 14th, the Ministry of Food and Drug Safety (MFDS) completed the safety and efficacy review of ‘Leqembi (lecanemab),’ a new drug for Alzheimer's disease that was co-
- Policy
- SGLT-2 monotherapy 'Suglat' set to withdraw from KOR
- by Lee, Tak-Sun May 13, 2024 05:51am
- The SGLT-2 inhibitor 'Suglat tab 50 mg (Ipragliflozin L-Proline),' which is used as monotherapy, will no longer be available in the market in South Korea. Three SGLT-2 monotherapies that were imported will be withdrawn from the Korean market, as Steglatro and Forxiga are set to be withdrawn. According to the Ministry of Food and Drug
- Policy
- 'BIO KOREA 2024' begins
- by Lee, Hye-Kyung May 10, 2024 05:47am
- 'BIO KOREA 2024' will be held on May 8-May10 for three days at Coex, Seoul. The conference is co-organized by the Korea Health Industry Development Institute (President Soon-do Cha, referred to as KHIDI) and Chungcheongbuk-do Province (Mayor Young Hwan Kim, referred to as Chungbuk), and sponsored by the Ministry of Health and Welfare (MOHW).
- Policy
- Celltrion Pharm completes localizing 3 Edarbi products
- by Lee, Hye-Kyung May 9, 2024 05:52am
- Celltrion Pharm has completed the localization of Edarbi Tab (azilsartan medoxomil potassium), a treatment for essential hypertension. On the 8th, the Ministry of Food and Drug Safety (MFDS) simultaneously withdrew and approved the authorization of Celltrion Pharm's Edarbi Tab 20mg. In December 2020, Celltrion Pharm acquired the entire
- Policy
- Name of transferred and aquired drugs can be changed in KOR
- by Lee, Hye-Kyung May 9, 2024 05:52am
- Korea’s regulations now stipulate that brand names of a drug transferred to a different company can be changed. According to industry sources on the 7th, the Ministry of Food and Drug Safety recently announced the revision of the 'Casebook for Drug Product Naming Practices (Citizen's Guide)' and announced the allowance of brand name changes.
- Policy
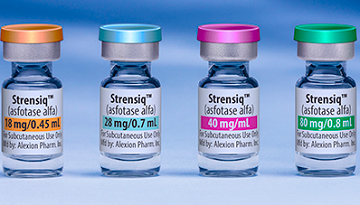
- AZ renews orphan drug Strensiq’s risk-sharing agreement
- by Lee, Tak-Sun May 9, 2024 05:52am
- The risk-sharing agreement (RSA) for Strensiq Inj (asfotase alfa, AZ), a treatment for hypophosphatasia, has been successfully renewed in Korea. The drug was last covered under the RSA scheme in June 2020, when it was listed for reimbursement in Korea under the Expenditure Cap Type and Refund Type RSA. According to the National Health