- Policy
- Reimb price of Forxiga generics fluctuates amid changes
- by Lee, Tak-Sun Apr 12, 2024 05:41am
- How the changes in reimbursement status of Forxiga (dapagliflozin propanediol monohydrate) generics will affect market competition is gaining industry-wide attention. The generic versions, which had been introduced to the market in April last year, are facing price changes due to changes in their reimbursement status. The premium pricing
- Policy
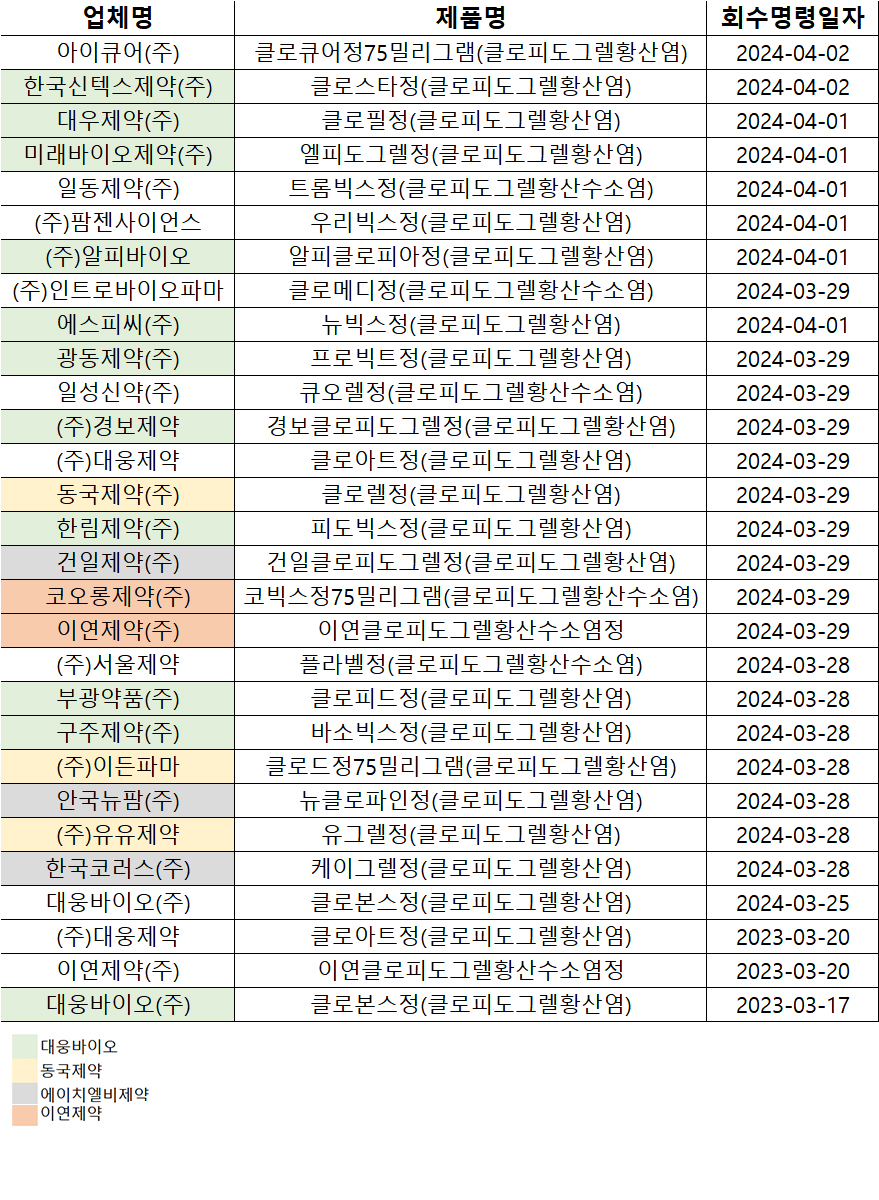
- Clopidogrel recall due to 1 CMO…’no issue escalation’
- by Lee, Hye-Kyung Apr 11, 2024 05:44am
- The issue that stirred up the clopidogrel recall that had been ongoing since March was found to have been caused by a single contract manufacturer, making the concern of it escalating to a series of recalls unlikely. Starting with Daewoong Bio's 'Clovons Tab' on March 17, the Ministry of Food and Drug Safety recalled a total of 29 items f
- Policy
- 4th-generation, 3 chamber IV nutrition receives reimb
- by Lee, Tak-Sun Apr 11, 2024 05:44am
- Fourth-generation 3 chamber Total Parenteral Nutrition (TPN), with enhanced amino acids contents, are being introduced into the market. Following the reimbursement listing of related products by Baxter and JW Pharmaceutical, HK inno.N and Fresenius Kabi have joined the competition. Companies that competed previously in the third-generat
- Policy
- Approval of Beyfortus imminent in Korea
- by Lee, Hye-Kyung Apr 9, 2024 05:50am
- Beyfortus (nirsevimab), a long-acting antibody designed to prevent respiratory syncytial virus (RSV) in infants that was jointly developed by Sanofi and AstraZeneca, is soon to receive marketing authorization in Korea. According to the minutes of the Central Pharmaceutical Affairs Council meeting held on March 6, which was released by the
- Policy
- 'Need patient engagement for reimb of high-priced drugs'
- by Lee, Tak-Sun Apr 8, 2024 05:46am
- A study has shown that there is a need for a formal process for patient organizations and patients to participate in discussions for the reimbursement of high-priced drugs in Korea. With the reimbursement of high-priced drugs rising as a social issue and patient organizations and others raising concerns, the opinion has risen on the need for
- Policy
- Recalls of antiplatelet drugs containing 'clopidogrel'…
- by Lee, Hye-Kyung Apr 5, 2024 05:44am
- The recall of the products due to exceeding safety standards for miscellaneous impurities in safety tests for the antiplatelet drugs containing the ingredient 'clopidogrel' is expanding. The Ministry of Food and Drug Safety (MFDS) reported that a total of 29 items have been recalled until April 2, starting with Daewoong Bio’s 'Clovons Tab'
- Policy
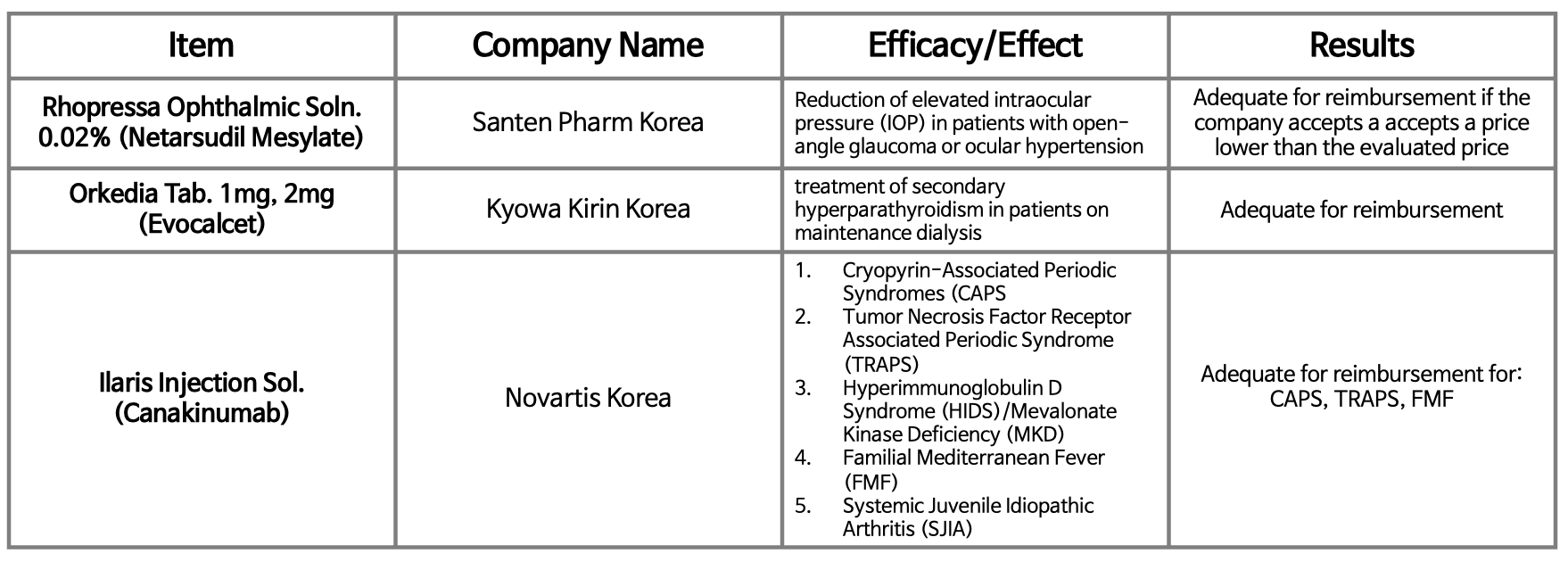
- Orphan drug Ilaris receives conditional pass for reimb again
- by Lee, Hye-Kyung Apr 5, 2024 05:43am
- Although the government restarted reimbursement discussions for Novartis Korea's orphan drug Ilaris Inj (canakinumab) after 2 months, the results were the same. According to the "Results of the 4th 2024 Drug Reimbursement Review Committee Deliberations," which was released on the 4th by the Health Insurance Review and Assessment Service, Ilar
- Policy
- HIRA in final stages of preparing expense report survey
- by Lee, Tak-Sun Apr 5, 2024 05:43am
- The Health Insurance Review and Assessment Service is busy preparing a survey and public disclosure of the expenditure reports on economic benefits pharmaceutical companies and medical device companies provided to doctors and pharmacists. As the data submitted by pharmaceutical companies through the survey will be subject to public dis
- Policy
- Trajenta generics enter reimbursement pricing…
- by Lee, Tak-Sun Apr 5, 2024 05:43am
- The generic version of Trajenta (linagliptin), a DPP-4 inhibitor class, has applied for reimbursement pricing ahead of its launch in June. This year, generic market is drawing attention to Trajenta. With the exclusive rights for Trajenta is set to expire, after Forxiga and Januvia last year, the diabetes market is expected to see new c
- Policy
- Eye drops late to submit test results pass reivew
- by Lee, Hye-Kyung Apr 4, 2024 05:59am
- Amid the ongoing equivalence reevaluations being conducted on eye drops, products that received dispositions for failing to submit equivalence data in time received a final compliant decision. The Ministry of Food and Drug Safety (MFDS) released the results of the ‘2022 drug equivalence reevaluation’ that contained such results on the 3rd.