- Policy
- Minister Cho will work to address shingles vaccine issue
- by Lee, Hye-Kyung Oct 26, 2023 06:06am
- The government responded that it would seek ways to ease the public burden in response to the request that shingles vaccines should be included in Korea’s National Immunization Program (NIP). Rep Young-Joo Kim of the Democratic Party of Korea pointed at the audit of the NA Health and Welfare Committee that was held on the 25th. She said,
- Policy
- Prostate cancer tx Xtandi·Zytiga, 5% pt copayment applied
- by Lee, Tak-Sun Oct 26, 2023 06:06am
- Mandatory coverage is applied to some treatments for prostate cancer treatments Xtandi soft capsule 40mg and Zytiga, which is expected to reduce the financial burden on patients. This is a measure of fairness as Erleada is subject to mandatory benefit as it was previously listed. The HIRA announced that it will revise the anticancer drug
- Policy
- Will restrictions be eased for vaccine clinical trials?
- by Lee, Jeong-Hwan Oct 26, 2023 06:06am
- Minister of Food and Drug Safety Yu-Kyung Oh promised to reduce or ease the mandatory participation rate of domestic clinical patients from the current 10% to 5% to strengthen sovereignty over domestically produced vaccines and increase self-sufficiency. The plan is to improve the current regulations by accepting the claims raised by dome
- Policy
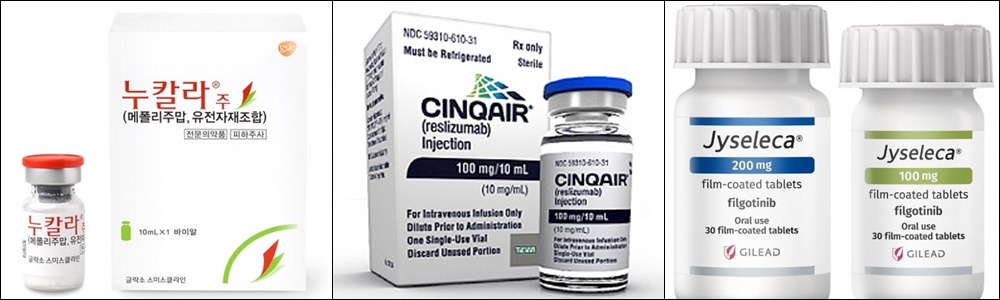
- Severe asthma txs Nucala and Cinqair are to be reimbursed
- by Lee, Jeong-Hwan Oct 24, 2023 05:22am
- Reimbursement treatment options for the treatment of severe eosinophilic asthma are expanded. GSK Korea's Nucala and Handok Teva Cinqair will be listed on the payroll simultaneously next month. Eisai's JAK inhibitor Jyseleca will also have new insurance coverage standards, and Novartis' JAK1/JAK2 inhibitor Jakavi will have coverage standards
- Policy
- TZD combined DPP4i-SGLT2i complex
- by Lee, Tak-Sun Oct 24, 2023 05:21am
- Due to the expansion of the reimbursement standards for combination diabetes drugs, combination drugs combining TZD-based ingredients with DPP4i or SGLT2i are expected to be released in November. Most of these products are combination drugs combining the DPP4i series Sitagliptin. According to the industry on the 23rd, TZD combination drugs s
- Policy
- Announcement of dual pricing system
- by Lee, Jeong-Hwan Oct 20, 2023 05:32am
- The Ministry of Health and Welfare is expected to include the application of a refund-type risk-sharing system for innovative new drugs in the plan to provide appropriate information on the innovative value of new drugs to be announced soon. In order to improve accessibility to rare disease treatments, the company announced its position to c
- Policy
- Will a new Alzheimer’s drug be introduced in 20 yrs in KOR?
- by Lee, Tak-Sun Oct 20, 2023 05:31am
- New Alzheimer's disease treatments are accelerating their introduction into the country. Following Eisai's application for the approval of its ‘lecanemab’ in June, Lilly also received approval from the Ministry of Food and Drug Safety to initiate a multinational Phase 3 clinical trial for ‘donanemab’ in Korea. As Biogen applied to
- Policy
- HIRA ‘will discuss reimb obesity treatments with MOHW'
- by Lee, Tak-Sun Oct 19, 2023 05:29am
- In response to the claim that obesity should be recognized as a chronic disease and its treatment covered by Korea’s health insurance, President Jung-gu Kang of the Health Insurance Review and Assessment Service responded that he would discuss the request with the Ministry of Health and Welfare. Kang made this announcement at the Natio
- Policy
- NA calls for prompt reimb of Ilaris during NA audit
- by Lee, Tak-Sun Oct 19, 2023 05:29am
- The National Assembly requested progress to be made in reimbursing ‘Ilaris (canakinumab, Novartis),’ a drug used to treat Hereditary recurrent fever syndromes that affect 13 patients in Korea. Rep. Sun-Woo Kang of the Democratic Party of Korea and member of the National Assembly Health and Welfare Committee suggested so at the Health I
- Policy
- Rep. Young-hee Choi ‘expenses surged after Moon Care’
- by Lee, Tak-Sun Oct 18, 2023 05:49am
- After the implementation of Moon Jae-in Care, which was implemented to strengthen health insurance coverage, KRW 5.272 trillion was found to have been spent on drugs for seriously ill patients over the 6 years and exceeded the expenditure target every year. According to data Rep. Choi Young-hee (People Power Party, proportional representa