- Policy
- Gov’s halts price cut discussions on generics
- by Lee, Jeong-Hwan Jul 10, 2023 05:21am
- Pharmaceutical companies in Korea are carefully monitoring the government's direction in improving the pricing system of generic drugs, including the government's insurance ceiling price policy. The Ministry of Health and Welfare was known to be contemplating a system with the health insurance authorities to further reduce the price of
- Policy
- Taiwanese new drug receives reimb in only 1 yr since release
- by Lee, Tak-Sun Jul 7, 2023 05:43am
- The Nephoxil Cap (ferric citrate hydrate) that was granted reimbursement at KRW 377 per capsule from the 1st of this month is actually a Taiwanese new drug. The drug, which is being imported and sold by the Japanese pharmaceutical company Kyowa Kirin’s Korean subsidiary in Korea, was developed by a Taiwanese pharmaceutical company. In ot
- Policy
- Evaluation different after Kymriah·Zolgensma benefits?
- by Lee, Tak-Sun Jul 7, 2023 05:43am
- The ultra-expensive treatments Zolgensma and Kymriah are supposed to go through a performance evaluation, but whether or not they are disclosed is different. As a pre-approved drug, Zolgensma, which is subject to review by an expert review committee, has performance evaluation results disclosed to the public, whereas Kymriah does not. Therefore,
- Policy
- Pres. Yoon urges swift legislation for remote treatment
- by Kang, Shin-Kook Jul 6, 2023 05:39am
- President Yoon Suk-yeol has instructed the Ministry of Health and Welfare to make every effort to pass the amendment of the Medical Service Act for the introduction of non-face-to-face treatment, which is currently under discussion in the National Assembly, raising hopes for its rapid legislation. President Yoon made these remarks on the 4th
- Policy
- Roche Korea, "No decision has been made to resupply Madopar
- by Lee, Tak-Sun Jul 6, 2023 05:39am
- Roche Korea recently announced that it has no plans to resupply Madopar, a Parkinson's disease treatment for which the grace period for the deletion of benefits was recently extended. It is explained that the headquarters did not make a decision to resupply, and that no agreement was signed with any organization for resupply. The extension of
- Policy
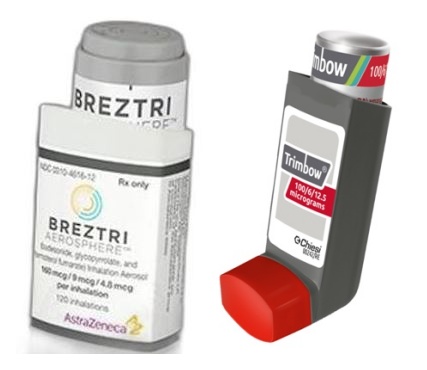
- Will AZ/Kolon succeed in challenging COPD combi benefit?
- by Lee, Tak-Sun Jul 5, 2023 05:45am
- Two COPD 3 drugs combi inhalers that are competing for reimbursement are not easily able to get out of the HIRA stage. AstraZeneca's 'Breztri Aerosphere' also requested a re-evaluation of the committee's results, while Kolon Pharmaceutical's 'Trimbow Inhaler' took on a re-challenge for reimbursement despite the drug evaluation committee's con
- Policy
- AZ Capivasertib, designated GIFT No. 8
- by Lee, Hye-Kyung Jul 5, 2023 05:45am
- On the 4th, the Ministry of Food and Drug Safety designated AstraZeneca Korea's breast cancer treatment Capivasertib as the 8th "Global Innovation Product Rapid Review (GIFT)." Starting with Lunsumio, a lymphoma treatment in Roche Korea, which was designated as GIFT No. 1 in November last year, it has been designated up to No. 8 recently, but no
- Policy
- Mounjaro, a weight loss effect for DM pts, has been approved
- by Lee, Hye-Kyung Jul 3, 2023 05:47am
- Eli Lilly's 'Mounjaro PFS', known overseas as a game changer for obesity treatment, has been approved in Korea. In Korea, it has been approved as a treatment for diabetes. Mounjaro is a synthetic peptide with a mechanism that can selectively bind to both the GIP and GLP-1 receptors for the first time in Korea. The Ministry of Food and Dru
- Policy
- Drug price negotiations such as Roche Evrysdi begin
- by Lee, Tak-Sun Jul 3, 2023 05:47am
- Roche's 5q spinal muscular dystrophy treatment Evrysdi Dry Syrup began negotiations with the NHIS as soon as it passed the Drug Benefit Evaluation Committee of the HIRA. Ace Pharma and H&O Pharm's multiple myeloma treatments Megval Inj. and Melpspal Inj. have also entered negotiations. The NHIS added oEvrysdi Dry Syrup 0.75 mg/mL, Melpspal In
- Policy
- Eval period for HCV treatment shortened to 12 weeks from 24
- by Lee, Hye-Kyung Jun 30, 2023 06:01am
- The evaluation period used to determine the efficacy of chronic hepatitis C treatments has been reduced in Korea. The Ministry of Food and Drug Safety (Minister Yu-Kyoung Oh) revised the 'Clinical Trial Evaluation Guidelines for Chronic Hepatitis C Treatments' on June 29 to align Korea’s clinical trial efficacy evaluation standards with