- Policy
- Drugs subject to 2nd evaluations may submit data by Oct
- by Lee, Tak-Sun Feb 9, 2023 05:50am
- Drugs that were to receive the 2nd series of reevaluations by HIRA and were required to submit data by July will also be deemed to have met their requirements even if they exceed their submission deadline and submit the Ministry of Food and Drug Safety review completion notice by the end of the objection period. This will allow companie
- Policy
- Enhertu's referral to the National Petition Welfare Committe
- by Lee, Jeong-Hwan Feb 9, 2023 05:49am
- It was referred to the National Assembly's Health and Welfare Committee as the number of people's consent to the application of breast cancer treatment Enhertu's health insurance reached 50,000. Enhertu is an antibody-drug conjugate that obtained a domestic marketing license last year due to the petition of the National Assembly. The petition
- Policy
- Legislation of health functional foods
- by Lee, Jeong-Hwan Feb 8, 2023 05:53am
- A bill to make drug preferential treatment for drugs developed by innovative pharmaceutical companies mandatory by law and a bill to institutionalize customized health functional foods will be presented to the plenary session of the National Assembly's Health and Welfare Committee on the 9th. A bill to strengthen regulations on specialized h
- Policy
- MFDS prepares a list of pre-requisite data for inspections
- by Lee, Hye-Kyung Feb 7, 2023 05:47am
- The Ministry of Food and Drug Safety has prepared a list of pre-requisite data for local inspections of overseas pharmaceutical manufacturing plants. A total of seven data submission lists, including the list of drug manufacturing over the past three years, have been prepared to enhance the predictability and consistency of administration dur
- Policy
- Further proposals made for reimb of diabetes combos
- by Lee, Tak-Sun Feb 7, 2023 05:47am
- The health insurance authorities have once again started analysis on the fiscal impact of reimbursing diabetes combination drugs. In the latest financial impact analysis, a red light was turned on in reimbursing the combined use of diabetes drugs as the reimbursement amount exceeded the government’s range of expectations. Whether the ne
- Policy
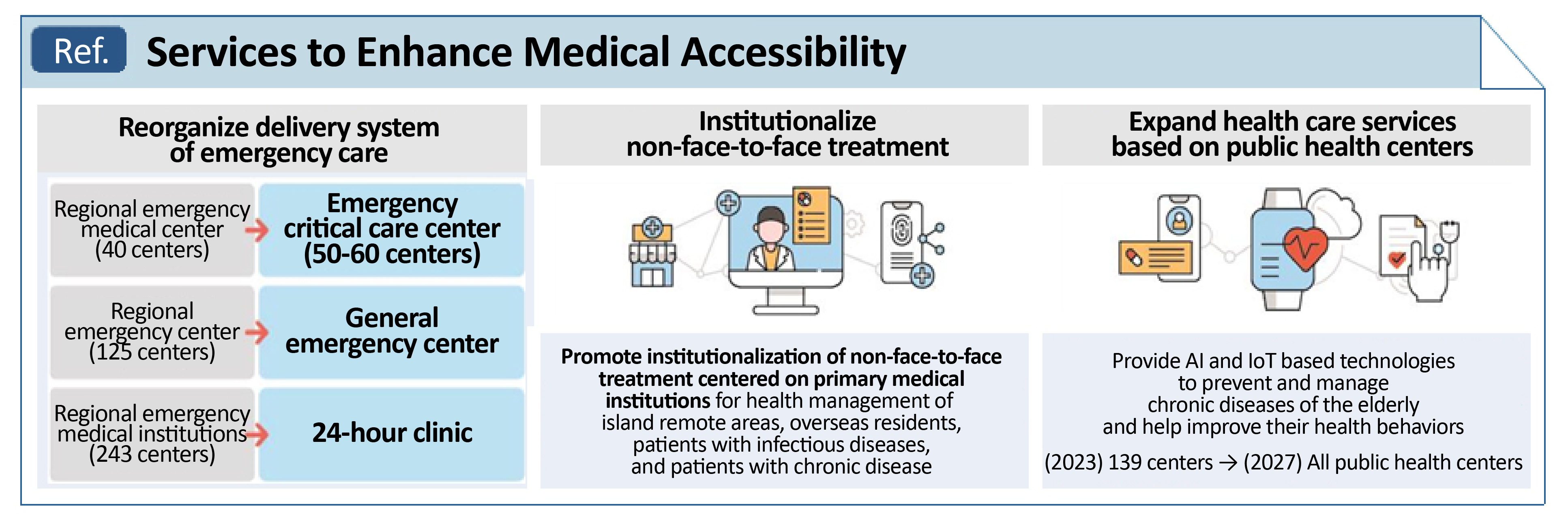
- Non-face-to-face treatment included as key policy task
- by Kang, Shin-Kook Feb 7, 2023 05:47am
- The Korean government is expected to make systemic improvements in the field of healthcare this year, with non-face-to-face treatment included as a key social policy. The Ministry of Education (Deputy Prime Minister and Education Minister Ju-Ho Lee) held the first Ministerial Conference on Social Relations at the Government Complex-Seoul on t
- Policy
- The price of AbbVie Skyrizi is likely to be adjusted
- by Lee, Tak-Sun Feb 6, 2023 05:51am
- AbbVie's moderate-severe psoriasis treatment Skyrizi PFS has agreed to a PVA negotiation, which is expected to reduce the upper limit. The drug was registered in June 2020, and it is analyzed that it exceeded the expected claim agreed in advance with the NHIS from 2021. According to the industry on the 3rd, the NHIS has completed PVA negotiat
- Policy
- The aftermath of the COVID-19 Pandemic
- by Lee, Hye-Kyung Feb 3, 2023 05:54am
- There is a need to convert COVID-19 vaccines and treatments, which had been designated for rapid screening, to general screening to cope with the COVID-19 pandemic situation. This is because it is argued that screening personnel should not be wasted due to rapid screening of COVID-19 vaccines and treatments in a situation where the world is conc
- Policy
- CDDC sets reimb standards for Inrebic Capsule
- by Lee, Tak-Sun Feb 3, 2023 05:54am
- A green light has been turned on for the reimbursement of the myelofibrosis treatment Inrebic Cap. (fedratinib, BMS Pharmaceutical Korea) with the Cancer Disease Deliberation Committee setting reimbursement standards for the drug. Also, the reimbursement standards for the ovarian cancer treatment Zejula Cap (niraparib, Takeda Pharmaceutical)
- Policy
- Introduction of online electronic sign-off from February
- by Lee, Tak-Sun Feb 2, 2023 05:48am
- NHIS Pharmaceutical Price Negotiation Introduces Online Electronic Signing from February Simplify operations to shorten the end of negotiations. The online electronic signing method will be introduced in next month's drug price negotiations. It is expected to simplify work and shorten the negotiation period by compensating for the shortcomin