- Company
- Immuno-oncology drugs demonstrate additional benefit at ASCO
- by Son, Hyung-Min Jun 7, 2024 05:50am
- Global pharmaceutical companies have demonstrated their immuno-oncology drugs’ effect in refractory solid tumors. Clinical results from major immuno-oncology drugs, including Imfinzi, Opdivo, and Yervoy, were presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, which kicked off last month in Chicago, U.S.
- Company
- "Breast cancer treatments received positive reviews"
- by Son, Hyung-Min Jun 7, 2024 05:50am
- Major antibody-drug conjugates (ADC) have secured positive clinical results in treating breast cancer. ADCs under development by global pharmaceutical companies have shown to be effective in various types of breast cancer, including triple-negative breast cancer, hormone-positive (HR+)/HER2-negative breast cancer. With these results, latecom
- Company
- ‘Fully utilize the various IBD treatment options available'
- by Eo, Yun-Ho Jun 7, 2024 05:50am
- Incidence of Inflammatory bowel disease (IBD), which had been regarded as a condition typically associated with Westerners, is now rising amongst Asian populations as well, including Koreans. According to the IBD fact sheet published by the Korean Association for the Study of Intestinal Diseases, the number of patients with ulcerative col
- Company
- ADC·immunotherapy competitiveness↑…K-bio gains attention
- by Son, Hyung-Min Jun 7, 2024 05:50am
- Korea-based biopharmaceutical companies have confirmed clinical achievements in globally trending R&D areas, including antibody-drug conjugates (ADC), immunotherapy for cancer, and bispecific antibodies. LigaChem Biosciences presented clinical data of LCB14, a human epidermal growth factor receptor 2 (HER2)- targeting ADC candidate. LBC1
- Company
- ‘Hemlibra is the most advanced hemophilia A drug availble'
- by Nho, Byung Chul Jun 5, 2024 05:47am
- "Hemlibra has the potential to become the standard of care for hemophilia A, and is considered the most advanced treatment available." Dr. Midori Shima, professor of pediatric hematology-oncology at Nara Medical University in Japan, said so at a media session to mark the 1st anniversary of Hemlibra's insurance reimbursement extension at
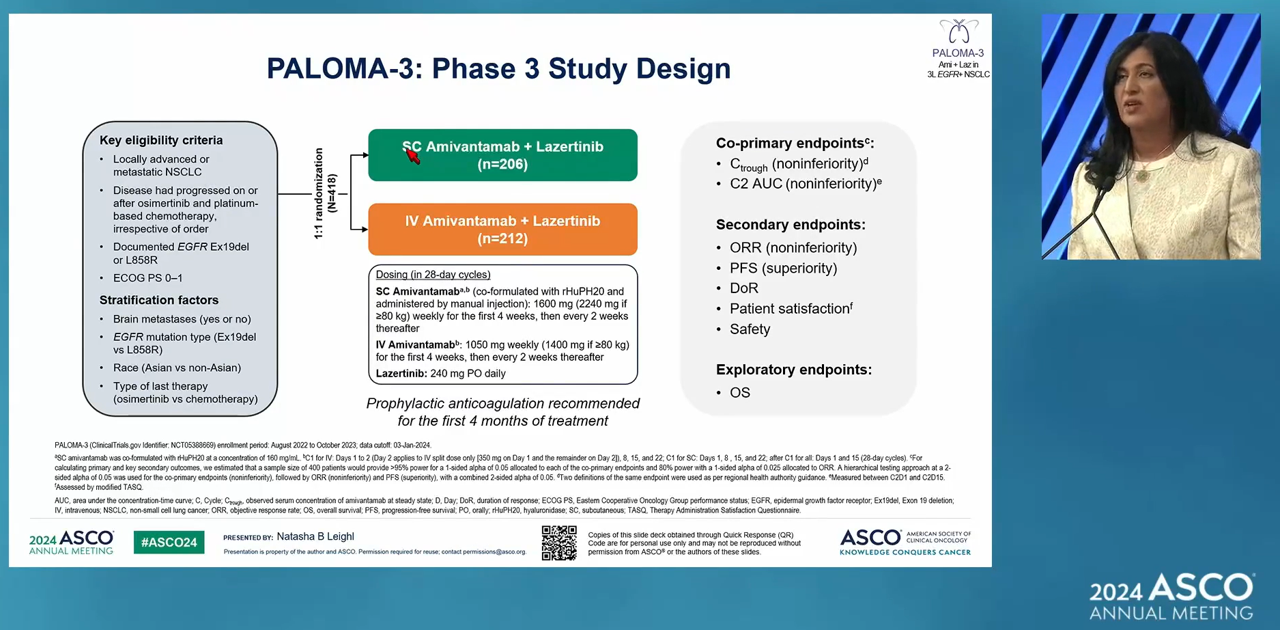
- Company
- More data on Leclaza·Lorviqua showcased
- by Son, Hyung-Min Jun 5, 2024 05:47am
- Positive clinical results for targeted therapies, such as EGFR and ALK, were featured at the international conference. Clinical achievements of major targeted therapies for non-small cell lung cancer (NSCLC), including Leclaza, Rybrevant, and Lorviqua, were presented at the American Society of Clinical Oncology (ASCO 2024) annual meeting in Chic
- Company
- Sprycel patent to expire soon…market shift worth KRW 40 bil
- by Nho, Byung Chul Jun 4, 2024 05:48am
- Following the patent expiration for blockbuster medicines in the United States this year, generics are expected to enter the market. Likewise, market shifts are expected with the development and launch of generics in South Korea. According to an overseas research agency, the top 10 blockbuster medicines with patents expiring in 2024 inc
- Company
- Indication for rare cancer drug Retevmo is expanded
- by Son, Hyung-Min Jun 4, 2024 05:47am
- The indication for Retevmo, which targets a rare disease mutation in lung cancer, has been expanded to include children with solid tumors across the spectrum. Retevmo is approved in South Korea for lung cancer but not reimbursed in Korea. It will be interesting to see if the additional confirmation of clinical efficacy will have an impact on
- Company
- Evolution of ADCs… Enhertu prolongs OS in solid tumors
- by Son, Hyung-Min Jun 4, 2024 05:47am
- The antibody-drug conjugate (ADC) anticancer drug Enhertu has demonstrated further efficacy across multiple solid tumors, including HER2-low breast, biliary tract, and head and neck cancers. With the clinical results, the company secured momentum to add more solid tumors to Enhertu’s already established breast, gastric, and non-small cell
- Company
- 'Jemperli,' a PD-1 inhibitor immunotherapy lands in Big 5
- by Eo, Yun-Ho Jun 4, 2024 05:46am
- 'Jemperli,' the first immunotherapy for endometrial cancer, has landed in Big 5 general hospitals. According to industry sources, GSK Korea’s PD-1 inhibitor Jemperli (dostarlimab) has cleared the Drug Committee (DC) of the nationwide medical institutes, including Samsung Medical Center in Seoul, Seoul National University Hospital, Seo