- Company
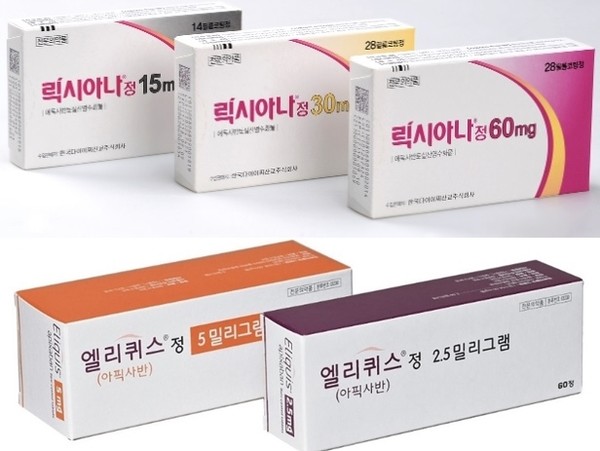
- Series of drug patent expiries change ₩250B DOAC mkt
- by Moon, sung-ho May 21, 2024 05:55am
- The direct oral anti-coagulant (DOAC) market is changing shape at an accelerated pace. This is due to the series of patent expiries of original drugs that had recorded high sales, mainly in the cardiology departments of university hospitals and clinic-level medical centers. In particular, with the accelerated entry of generic drugs (g
- Company
- Rolvedon's cumulative US sales surpasses ₩100B
- by Son, Hyung-Min May 21, 2024 05:55am
- Hanmi Pharmaceutical's neutropenia drug Rolvedon is cruising in the US market. Rolvedon has surpassed KRW 100 billion in cumulative sales in the US market. Hanmi Pharmaceutical's U.S. partner Assertio plans to further increase the drug’s market share by securing a same-day dosing indication. According to Assertio on the 18th, Rolvedon genera
- Company
- Dong-A, Ildong join hands…R&D synergy and resources
- by Chon, Seung-Hyun May 21, 2024 05:55am
- Dong-A ST and Idience join hands to develop novel anticancer drugs. Dong-A ST invested KRW 25 billion in Ildong’s subsidiary, Idience, and will co-develop novel anticancer drugs. Consequently, Dong-A ST has secured a pipeline of potential novel anticancer drugs, and Idience successfully contracted investment for its novel drug development.
- Company
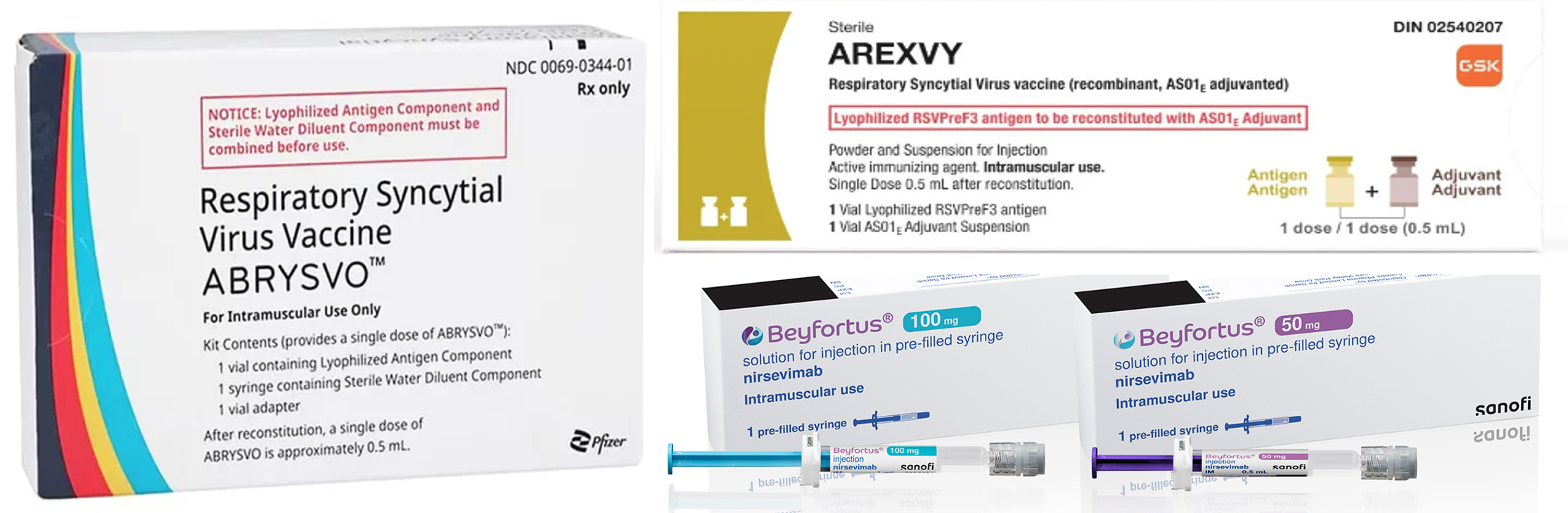
- Global RSV vaccines emerge…K-pharma enters clinical trials
- by Son, Hyung-Min May 20, 2024 05:42am
- Competition is expected to intensify as new drugs emerge in the respiratory syncytial virus (RSV) market. Sanofi received approval for its RSV vaccine and is the third company to acquire the approval, following GSK and Pfizer. Furthermore, Moderna’s messenger RNA (mRNA) vaccine awaits the FDA approval. In South Korea, EuBiologics has ente
- Company
- ‘Much work remains to be done for new drug access in Korea'
- by Eo, Yun-Ho May 20, 2024 05:42am
- Kyung-Eun Bae (Kay&160;Bae, 53) has risen to become the 'center' of the pharmaceutical industry. After being appointed as the general manager to lead subsidiaries including Korea, New Zealand, and Australia of the Sanofi Group, Bae was also appointed Chair of the Korean Research-based Pharmaceutical Industry Association (KRPIA) Being app
- Company
- Welireg can be prescribed in general hospitals in Korea
- by Eo, Yun-Ho May 20, 2024 05:41am
- Welireg, a rare anticancer drug for a small number of patients, can now be prescribed in general hospitals in Korea. According to industry sources on the 17th, MSD Korea’s oral hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor, ‘Welireg (belzutifan) passed the drug committees (DCs) of various medical centers in Korea including the Na
- Company
- 'Imfinzi' shown to be effective for liver cancer
- by Son, Hyung-Min May 17, 2024 05:47am
- Imfinzi, an immunotherapy treatment for cancer, demonstrated effectiveness for liver cancer in addition to bile duct cancer. Consequently, it is expanding its uses in gastrointestinal cancer. AstraZeneca confirmed the effectiveness of Imfinzi in combination therapy with Imjudo, which targets CTL-A4, as a first-line treatment for liver canc
- Company
- Ferring Korea appoints Min-Jung Kim as new GM
- by Son, Hyung-Min May 17, 2024 05:47am
- On May 16, Ferring Pharmaceuticals Korea announced that the company appointed Min-Jung Kim as its new general manager, effective as of May 1. The new GM joined the company in 2021 as Chief Financial Officer (CFO). Kim has over 20 years of experience in finance, business development, SCM, IT, and human resources at global pharmaceutical an
- Company
- Repotrectinib receives orphan drug designation in Korea
- by Eo, Yun-Ho May 17, 2024 05:47am
- , The &160;ROS1-targeted lung cancer drug repotrectinib received an orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) announced the designation in a recent announcement. Specifically, the indications repotrectinib received orphan drug designation for are as follows: ▲ treatment for patients with ROS1-posit
- Company
- Chinese Pharmas show presence at BIO Korea 2024
- by Moon, sung-ho May 16, 2024 05:48am
- Chinese pharmaceutical companies are starting to enter the domestic pharmaceutical and biotechnology market in earnest. This was the main observation made by the Korean industry at ‘BIO Korea 2024,’ cohosted by the Ministry of Health and Welfare and the Korea Health Industry Development Institute. The activities of major Chinese phar