- Company
- SK Bioscience breaks ground on vaccine plant expansion
- by Chon, Seung-Hyun Mar 11, 2024 05:55am
- SK Bioscience announced on the 7th that the company broke ground on a vaccine plant, ‘Andong L HOUSE,’ located in Andong, Gyeongsangbuk-do, South Korea. The plant will be expanded to install new equipment. This expansion aims to increase vaccine production capacity for global supply by raising the existing vaccine plant within L House
- Company
- Jemperli can be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Mar 11, 2024 05:55am
- Jemperli, the first immuno-oncology option introduced to the field of endometrial cancer, can now be prescribed in general hospitals in Korea. According to industry sources, GSK Korea’s PD-1 inhibitor Jemperli (dostarlimab) has passed the drug committee (DC) reviews of tertiary hospitals in Korea. Including the Seoul National University
- Company
- Hemlibra shows big sales rise, Advate loses ground
- by Kim, Jin-Gu Mar 11, 2024 05:55am
- The market for Type A hemophilia has shifted significantly, with JW Pharmaceutical's 'Hemlibra' expanding sales considerably after receiving reimbursement expansion. However, Takeda Pharmaceutical’s 'Advate,' which has been recording the highest sales in the market for an extended period, lost ground. Advate sales decreased by over 20%
- Company
- AZ’s EGPA drug Fasenra receives orphan drug designation
- by Eo, Yun-Ho Mar 11, 2024 05:55am
- AstraZeneca's antibody drug Fasenra has been designated as an orphan drug in Korea for its eosinophilic granulomatosis indication. The Ministry of Food and Drug Safety announced so through an official orphan drug designation notice on the 7th. More specifically, the drug received an orphan drug designation as a treatment for eosinophilic
- Company
- Anemia drug ‘Vadanem’ makes 2nd attempt at reimb listing
- by Nho, Byung Chul Mar 8, 2024 05:18am
- Vadanem, Mitsubishi Tanabe Pharma’s new tablet anemia drug, will likely receive approval from the U.S. Food and Drug Administration by early April and make a second attempt at getting a reimbursement listing in Korea. If the company gets the drug reasonably priced comparable to a weighted average price of the substitute during drug pri
- Company
- Oral PNH drug Fabhalta to soon be released in Korea
- by Eo, Yun-Ho Mar 7, 2024 07:15am
- Novartis' new PNH drug Fabhalta is set to land in Korea. According to industry sources, Novartis Korea has submitted an application for the marketing authorization of its Paroxysmal Nocturnal Hemoglobinuria (PNH) drug Fabhalta (iptacopan) to the Ministry of Food and Drug Safety. Therefore, the drug is expected to be approved within this
- Company
- Rare drug Ultomiris posts KRW 40 billion in sales in 3 yrs
- by Nho, Byung Chul Mar 6, 2024 06:03am
- Becoming a KRW 40 billion blockbuster in just 3 years since its launch, the growth potential of AstraZeneca’s Ultomiris Inj (ravulizumab) is gaining industry-wide attention. The biological orphan drug Ultomiris Inj. is considered to be the successor to Soliris Inj. (eculizumab) with significant improvements in dosing convenience. T
- Company
- Reimb for Verzenio in early breast cancer undergoes review
- by Eo, Yun-Ho Mar 6, 2024 06:03am
- Verzenio, the first CDK4/6 inhibitor to apply for insurance reimbursement in early breast cancer, is taking the second step to extend its reimbursement coverage. According to a report, the agenda of reimbursing Lilly Korea’s Verzenio (abemaciclib) will be presented to the Health Insurance Review and Assessment Service's Cancer Disease De
- Company
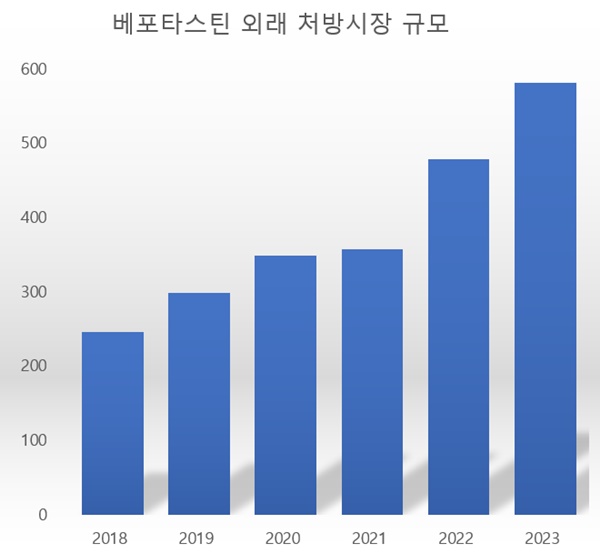
- Cash cow ‘bepotastine’ to face reimb re-evaluation
- by Chon, Seung-Hyun Mar 6, 2024 06:02am
- In facing re-evaluation of ‘bepotastine’ for reimbursement in the upcoming year, pharmaceutical companies fear its impact. The market size for bepotastine has increased by more than 50% in just two years during the pandemic and endemic, labeling bepotastine as a new cash cow. However, there are concerns that the companies may face financial lo
- Company
- Dupixent, 1st biologic approved for pruritic rash indication
- by Son, Hyung-Min Mar 5, 2024 05:49am
- As Dupixent is approved to treat prurigo nodularis (nodular itchy rash), the drug emerged as the only available treatment option among biologic agents. Previously, there were limited treatment options for treating prurigo nodularis, a condition that causes extreme itchiness. Sanofi hosted a press conference at Novotel Ambassador Seoul