- Company
- Kolon Life Science appeals to the Supreme Court for Invossa
- by Nho, Byung Chul Mar 5, 2024 05:49am
- On the 28th, Kolon Life Sciences decided to appeal to the Supreme Court against the manufacturing and sales license revocation ruling that had been made for its knee osteoarthritis cell gene therapy Invossa-K Inj (“Invossa”). In its appeal to the court, Kolon Life Sciences explained, "While we respect the court's decision, we will str
- Company
- Hugel receives FDA approval for its botulinum toxin Letybo
- by Nho, Byung Chul Mar 5, 2024 05:48am
- On the 4th, Hugel, a global total medical aesthetics company, announced that the company has received marketing approval from the U.S. Food and Drug Administration (FDA) on February 29th for 50 units and 100 units of its botulinum toxin Letybo (Korean brand name: Botulax). The FDA approval of Letybo represents a strong affirmation of Hu
- Company
- New multiple myeloma Ab ‘Elrexfio’ expects to enter KOR
- by Eo, Yun-Ho Mar 5, 2024 05:48am
- ‘Elrexfio,’ a new bispecific antibody to treat multiple myeloma, is expected to become commercially available soon. Pfizer Korea has applied for approval of Elrexfio (elranatamab) last year, and it is currently under review by the Ministry of Food and Drug Safety (MFDS), according to industry sources. Elrexfio is expected to be comme
- Company
- Intensifying competition in ulcerative colitis drug market
- by Son, Hyung-Min Mar 4, 2024 05:53am
- New drug development for conquering ulcerative colitis drives competition among global pharmaceutical companies. Competition in the market will likely intensify due to the efficacy shown by new drugs, including JAK inhibitors Rinvoq and Xeljanz, anti-integrin drugs, and S1P receptor modulators, in clinical trials, in addition to biological medic
- Company
- Generics of ‘Opsumit’ face tough competition in KOR
- by Kim, Jin-Gu Mar 4, 2024 05:52am
- In the market for the treatment of pulmonary arterial hypertension (PAH) with the active ingredient macitentan, the period of priority of sale given to the first generic drugs is set to expire on the first of next month. While Janssen’s ‘Opsumit’ competes with Samjin Pharm’s ‘Masiten’ in the market, Daewoong Pharmaceutical’s newly l
- Company
- Bayer releases 2 new CVD drugs with reimb in KOR
- by Eo, Yun-Ho Mar 4, 2024 05:52am
- Bayer Korea, which has been slow to launch new drugs, is making a comeback, recently succeeding in reimbursing two of its cardiovascular drugs in Korea. According to industry sources, Bayer Korea launched its heart failure drug ‘Verquvo (vericiguat)’ with reimbursement on September 1 last year and its kidney disease drug ‘Kerendia (finere
- Company
- Hanmi Pharm ‘confirms the effect of SERM+Vitamin D combo’
- by Son, Hyung-Min Feb 29, 2024 06:03am
- Hanmi Pharmaceutical announced on the 27th that the results of big data-based research on 'SERM+Vitamin D combination drugs', including its own ‘RaboneD’, were published in the SCI-level international journal, 'Osteoporosis International'. RaboneD is an osteoporosis treatment developed by Hanmi Pharmaceutical that combines vitamin D
- Company
- Roche Korea appoints Ezat Azem as new CEO
- by Eo, Yun-Ho Feb 29, 2024 06:03am
- Roche Korea is welcoming its new CEO, who will take up the position after a two-month vacancy. According to an interview, Roche Korea recently appointed Ezat Azem as new CEO. Before his appointment in Korea, Ezat Azem was a General Manager in Roche’s Greece subsidiary. Ezat Azem joined Roche’s Israel subsidiary in 1997 and worked
- Company
- K-Bio’s potential rises in targeted anticancer therapy
- by Son, Hyung-Min Feb 29, 2024 06:03am
- The achievements made by the pharmaceutical and bio-industry companies in Korea in developing targeted antitumor therapies are being introduced at academic conferences overseas. iLeadBMS and PharosiBio have announced positive preclinical study results, and have received the green light to enter main clinical trials. The ESMO Targeted Anticanc
- Company
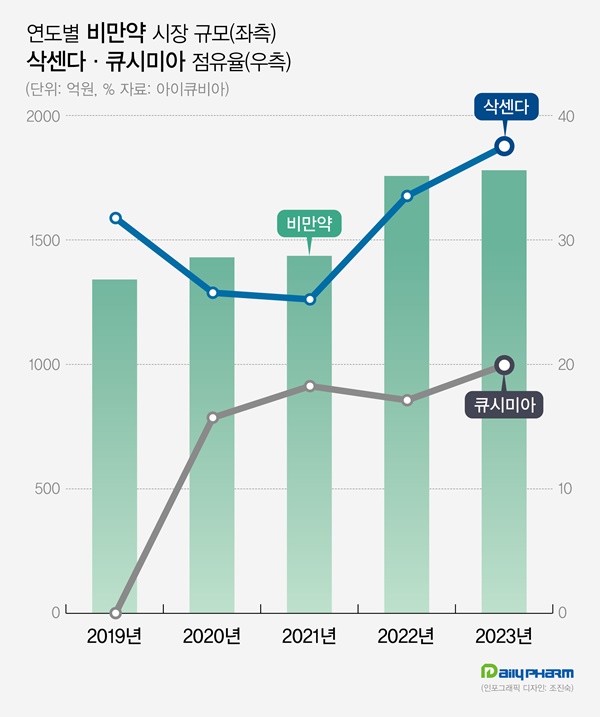
- Saxenda and Qsymia take up 57% of the obesity market share
- by Chon, Seung-Hyun Feb 28, 2024 10:35am
- Last year, Korea’s obesity drug market size reached its largest size in history. It broke the record in 2019, and since then, the market size expanded for five consecutive years. Saxenda and Qsymia account for 60 % of the total market share. Meanwhile, sales of previous obesity drugs, such as psychotropic drugs, are declining, further polarizin