- Company
- KRPIA appoints Sanofi CEO Kay Bae as new chair
- by Feb 28, 2024 05:50am
- The Korean Research-based Pharmaceutical Industry Association (KRPIA) announced on the 23rd that it has appointed Kay Bae (Bae Kyung-eun), Country Lead of Sanofi-Aventis Korea, as the 15th chair of KRPIA. Bae served on the KRPIA board of directors in September 2013, and she has been a member of the vice-chair body, contributing to the g
- Company
- Immuno-oncology market triples in 4 years
- by Son, Hyung-Min Feb 28, 2024 05:50am
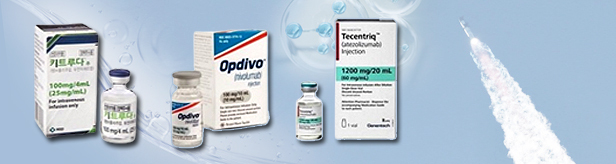
- Sales in the immuno-oncology market surpassed KRW 700 billion last year, driven by the surge in sales of Keytruda and Opdivo. The market has more than tripled in size over the past 4 years. Keytruda and Opdivo together accounted for 72.4% of the market and generated more than KRW 500 billion in sales. The market’s prospects are also bright with
- Company
- Will the first oHCM drug Camzyos be reimbursed in Korea?
- by Eo, Yun-Ho Feb 28, 2024 05:50am
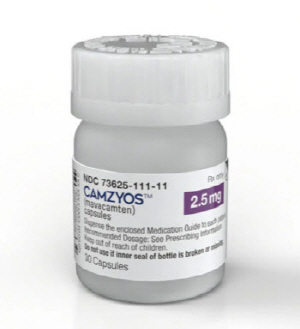
- The industry’s eyes are focused on the reimbursement review progress of Camzyos, the first treatment for obstructive hypertrophic cardiomyopathy. Dailypharm’s coverage found that the pharmacoeconomic evaluations for Camzyos(mavacamten), BMS Pharmaceutical Korea's new drug for obstructive hypertrophic cardiomyopathy (oHCM), is now comple
- Company
- Samsung Bioepis confirms efficacy of its Eylea biosimilar
- by Nho, Byung Chul Feb 27, 2024 05:45am
- Samsung Bioepis (CEO Hansung Ko) announced today that the company had presented the follow-up results from its Phase III clinical trial on SB15 (Korean brand name: Afilivu/Eylea biosimilar/Aflibercept) at the Annual Asia-Pacific Academy of Ophthalmology (APAO) Congress that was held in Indonesia from the 22nd to the 25th this month, SB1
- Company
- K-biopharma starts trials with bispecific antibodies
- by Son, Hyung-Min Feb 27, 2024 05:45am
- Major global pharmaceutical companies have accomplished successful marketing of bispecific antibodies. Now, the Korean biopharmaceutical industry is also joining this challenge. Current bispecific antibodies include Roche’s Lunsumio and Columvi, Abbvie’s Epkinly, and Janssen’s Tecvayli. Korean companies are planning to evaluate the possibilit
- Company
- Reimbursement for novel CMV drug Livtencity imminent in KOR
- by Eo, Yun-Ho Feb 27, 2024 05:45am
- The novel cytomegalovirus drug Livtencity will soon receive reimbursement in Korea. According to industry sources, Takeda Pharmaceutical Company of Korea recently completed drug pricing negotiations with the National Health Insurance Service for its cytomegalovirus (CMV) drug Livtencity (maribavir). The drug is expected to be approved in
- Company
- Shingrix leads shingles vaccine mkt…occupies 44% of mkt
- by Chon, Seung-Hyun Feb 26, 2024 05:24am
- The new shingles vaccine, Shingrix, has risen to lead the market in its first year of sales. It quickly gained 44% of the market share with its strong shingles prevention effect despite its high price. Since the addition of Shingrix, the market has doubled in size. According to the drug research institution IQVIA, the shingles vaccine market
- Company
- Samsung Bioepis’s Eylea biosimilar Afilivu approved in KOR
- by Son, Hyung-Min Feb 26, 2024 05:24am
- Samsung Bioepis announced on the 23rd that it has obtained domestic approval for its Eylea biosimilar ‘Afilivu.’ The approval marks the 2nd ophthalmic disease treatment Samsung Bioepis has received approval for after being granted approval for its Lucentis biosimilar Amelivu. Eylea is a macular degeneration treatment developed by the m
- Company
- Eylea continues to lead AMD mkt despite new competition
- by Son, Hyung-Min Feb 26, 2024 05:24am
- The entry of new drugs in the macular degeneration treatment market made little impact on Eylea’s sales. Eylea continued to top the macular degeneration treatment market last year, posting sales of KRW 96.7 billion. The Eylea biosimilars Vabysmo and Lucentis, which were released last year, have shown little presence in the market yet.
- Company
- Kay Bae appointed as KRPIA chair, also overseeing NZ, AUS
- by Eo, Yun-Ho Feb 26, 2024 05:24am
- Kay Bae (53), Sanofi-Aventis Korea Country Lead, literally has become the ‘center’ of the pharmaceutical company. Bae, who was recently appointed as a Country Lead to manage subsidiaries including Korea, New Zealand, and Australia of the Sanofi Group, is appointed as a new Chair of the Korean Research-based Pharmaceutical Industry Ass