- Company
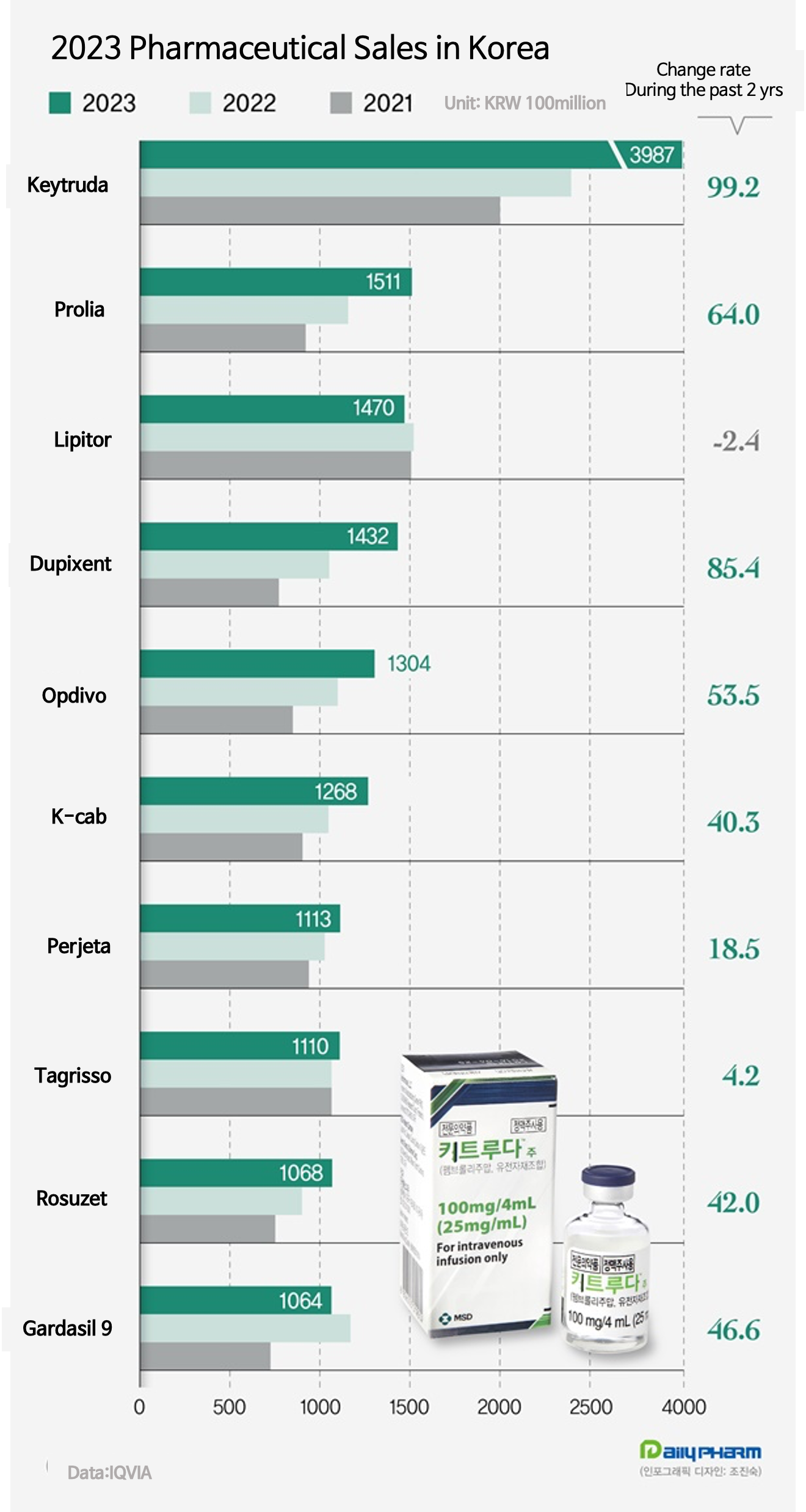
- Keytruda leads mkt for 4 consecutive years
- by Chon, Seung-Hyun Feb 21, 2024 05:45am
- The immuno-oncology drug Keytruda has taken the lead in the domestic market for the 4th consecutive year. Its quarterly sales exceeded KRW 100 billion for the first time in the domestic market after being expanded reimbursement to the first line. The gap with the runner-up had more than doubled, reinforcing Keytruda’s leadership in the market.
- Company
- Samsung Bioepis will directly sell 3 of its biosimilars
- by Nho, Byung Chul Feb 20, 2024 06:00am
- Samsung Bioepis is expected to strengthen its sales and marketing force and network and switch to direct domestic sales of its 3 self-developed biosimilars. According to industry sources, Samsung Bioepis will directly sell 3 of its biosimilars for autoimmune diseases (TNF-alpha inhibitors) in the domestic market from next month (March).
- Company
- Generic Pazeo sales 24%↑ after patent dispute win
- by Kim, Jin-Gu Feb 20, 2024 06:00am
- Last year, generic products in the market for eye drops with the active ingredient olopatadine, used to treat allergic conjunctivitis, posted a 24% year-over-year (YoY) increase in prescription sales. It is the most significant sales expansion since 2019. The pharmaceutical industry anticipates a substantial expansion in prescription sales o
- Company
- K-Pharma Bios jump into cancer vaccine development
- by Son, Hyung-Min Feb 19, 2024 05:45am
- Cancer vaccine candidates produced by domestic pharmaceutical and biotech companies are now one step closer to conquering intractable cancers, demonstrating effect in clinical trials. DXVX's cancer vaccine candidate has shown an effect on ovarian cancer and lung cancer, and Aston Science has entered Phase II clinical trials for gastric can
- Company
- Clopidogrel mkt exceeds KRW 500 bil for the first time
- by Kim, Jin-Gu Feb 19, 2024 05:45am
- The antiplatelet market for clopidogrel has grown to exceed KRW 500 billion in Korea. Although 25 years have passed since the original drug, Sanofi's Plavix, was released in Korea, its market is still showing growth. Last year, the growth of generic products stood out in the market. The combined prescription sales of generic products incre
- Company
- K-made new stroke drugs accelerate towards commercialization
- by Son, Hyung-Min Feb 19, 2024 05:45am
- New candidate products for ischemic stroke developed by Korean pharmaceutical companies are proving to be effective. As global pharmaceutical companies have faced difficulties in development, attention is drawn to the possibility of commercializing new stroke drugs made in Korea. According to industry experts on the 17th, Phase 3 clinical dem
- Company
- Can we expect Darzalex to receive expanded reimb this year?
- by Eo, Yun-Ho Feb 19, 2024 05:45am
- A multiple myeloma drug, ‘Darzalex,’ garners attention as to whether it could receive expanded use this year. Janssen Korea’s Darzalex (daratumumab) is under discussion for expanding reimbursement into multiple indications. It is categorized into two types of therapies. The first is the DVTd (daratumumab, bortezomib, thalidomide, a
- Company
- Administration cycle restriction on Xospata will be lifted
- by Eo, Yun-Ho Feb 19, 2024 05:44am
- The reimbursement restrictions set on the number of administration cycles set for the acute myeloid leukemia treatment 'Xospata' is expected to be lifted soon. According to Dailypharm’s coverage, Astellas Korea recently finalized drug pricing negotiations with the National Health Insurance Service to expand reimbursement coverage for its
- Company
- Keytruda sales expected to surpass 300 billion won
- by Nho, Byung Chul Feb 16, 2024 06:25am
- Keytruda holds a blockbuster position in the immune checkpoint inhibitors sector, with analysts expecting its sales to surpass 300 billion won. According to the distribution performance report, MSD’s Keytruda recorded 289.8 billion won, leading its sector. It was followed by BMS-Ono’s Opdivo, which accumulated 99.3 billion won up to 3
- Company
- Sobi, Handok’s partner has built competitive pipelines
- by Son, Hyung-Min Feb 16, 2024 06:03am
- Sobi, a biopharmaceutical company headquartered in Sweden, announced that it will launch its new drugs for treating rare diseases, including primary hemophagocytic lymphohistiocytosis (HLH), immune thrombocytopenia (ITP), and alkaptonuria (AKU), in Korea. Sobi and Handok have entered into a business agreement to launch in Korea. According to