- Company
- SK Biopharm’s new drug tops 500 bln won in US sales
- by Kim, Jin-Gu Feb 1, 2024 12:39pm
- SK Biopharmaceuticals’ Cenobamate, a new epilepsy drug, has generated cumulative sales of 500 billion won in the United States since its launch four years ago. Previously, SK Biopharmaceuticals struggled with operating losses almost every quarter, except for occasional profits from technology exports. However, the company has now turne
- Company
- Oral plaque psoriasis drug Sotyktu in final stages of reimb
- by Kim, Jin-Gu Feb 1, 2024 12:38pm
- The oral plaque psoriasis treatment ‘Sotyktu’ has entered its final stages to reimbursement in Korea. According to Dailypharm’s coverage, BMS Korea accepted the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee’s deliberation results and accepted the condition of setting the price of its TYK2 in
- Company
- HK Inno.N will copromote Xigduo and Sidapvia with AZ
- by Kim, Jin-Gu Feb 1, 2024 12:38pm
- HK Inno.N and AstraZeneca Korea announced on the 31st that they held a signing ceremony at HK Inno.N's Seoul office to mark their strategic collaboration of their diabetes portfolio. Under the agreement, HK Inno.N will jointly market and sell Xigduo (dapagliflozin + metformin) and Sidapvia (dapagliflozin + sitagliptin) with AstraZeneca Ko
- Company
- 13 of Keytruda’s indications await ‘redeliberation’
- by Eo, Yun-Ho Feb 1, 2024 12:38pm
- The first result for the reimbursement for the remaining 13 indications of Keytruda was ultimately a collective ‘hold.’ However, the reimbursement journey for 'Keytruda' has just begun. The Health Insurance Review and Assessment Service held its first Cancer Disease Deliberation Committee meeting of the new year on January 31st and revi
- Company
- MSD’s rare cancer drug Welireg first prescribed in KOR
- by Eo, Yun-Ho Jan 29, 2024 06:05am
- A rare anti-cancer drug targeting a very small number of patients, Welireg, has been prescribed in Korea. According to industry sources, the prescription code for MSD Korea’s oral hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor, ‘Welireg (belzutifan),’ has been registered at the Samsung Medical Center in Seoul. Currently, SMC is t
- Company
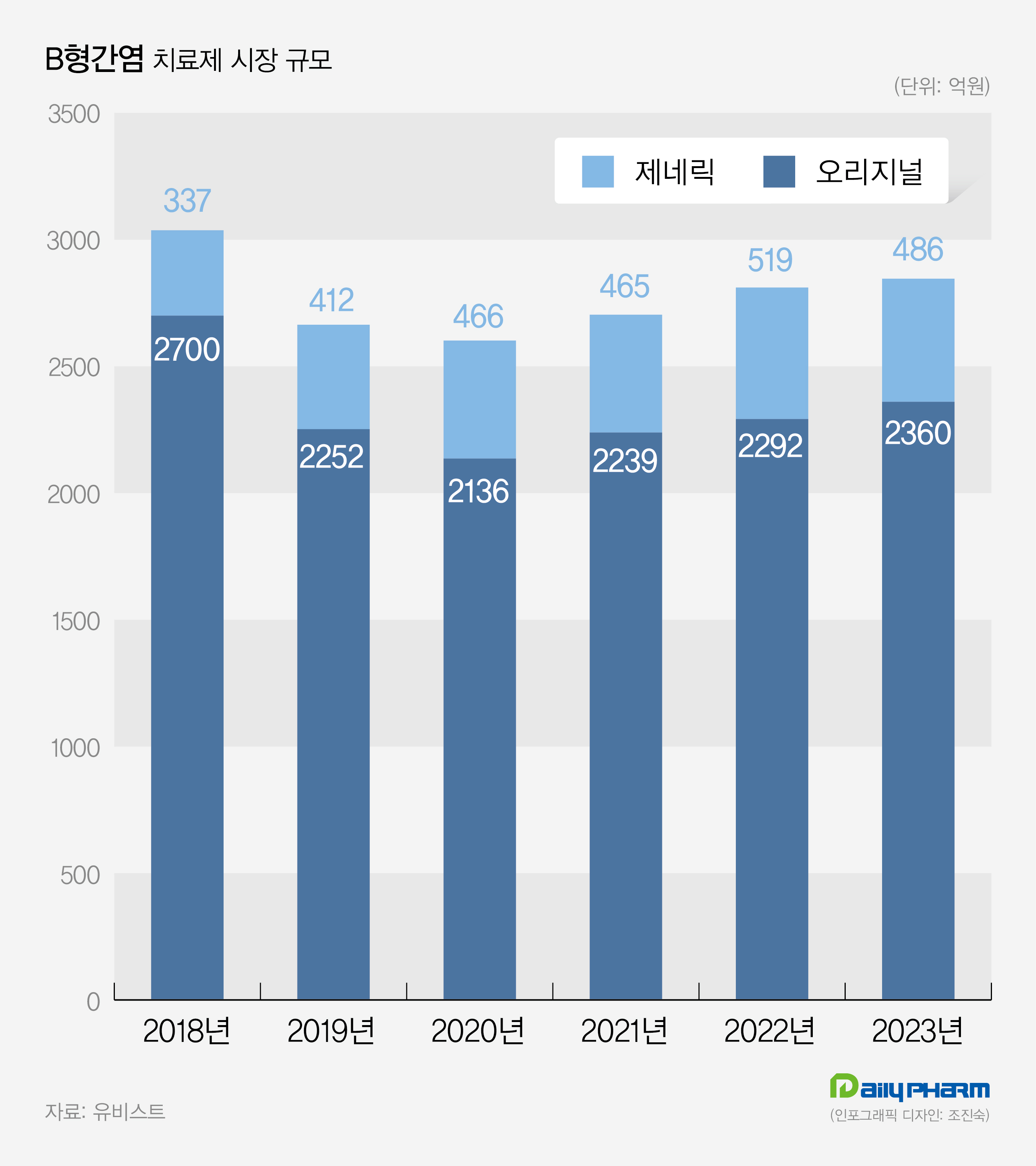
- Vemlidy dominates stagnant 300 bil. won hepatitis B market
- by Son, Hyung-Min Jan 29, 2024 06:05am
- The market for hepatitis B treatment, worth 300 billion won annually, is currently experiencing a slowdown. While Gilead Sciences Korea’s Vemlidy is expanding its market share, Viread and Baraclude are facing a decline. In the generics market, Dong-A ST is leading the market. Prescription sales for Vemlidy has increased by 26% compared t
- Company
- Will Polivy secure reimbursement approval this year?
- by Eo, Yun-Ho Jan 29, 2024 06:04am
- The question of whether ‘Polivy,’ a B-cell lymphoma treatment, will be listed for insurance reimbursements this year is garnering significant attention. According to industry sources, Roche Korea’s Polivy (polatuzumab vedotin), a treatment for relapsed or refractory diffuse large B-cell lymphoma (DLBCL), is expected to be presented to
- Company
- New CKD drug Kerendia lands in general hospitals in KOR
- by Eo, Yun-Ho Jan 26, 2024 05:51am
- The landing procedure for the new chronic kidney disease drug 'Kerendia' is in full swing in general hospitals in Korea ahead of its reimbursement listing. According to industry sources, Bayer Korea's Kerendia (finerenone) recently passed the drug committee (DC) review at Sinchon Severance Hospital. It is also undergoing a landing proces
- Company
- Ildong Idience presents 1st interim results for Venadaparib
- by Kim, Jin-Gu Jan 25, 2024 05:50am
- On the 22nd, Idience, the new drug development subsidiary of Ildong Pharmaceutical, announced that they have presented the research findings related to 'Venadaparib' at the 2024 ASCO Gastrointestinal Cancers Symposium held from the 18th to the 20th. Venadaparib is a novel targeted anticancer candidate product with a mechanism focused on
- Company
- Boehringer Ingelheim Korea appoints Ana-Maria Boie as new GM
- by Son, Hyung-Min Jan 25, 2024 05:49am
- Boehringer Ingelheim Korea announced on the 24th that it had appointed Ana-Maria Boie as the General Manager and Head of Human Pharma. The new GM, Ana-Maria Boie, is a seasoned expert with 24 years of experience in the pharmaceutical industry, in various areas including management, marketing, sales, and ESG. Boie joined Boehringer Ingelhe