- Company
- Samsung Biologics tops 1 trillion won in profit
- by Chon, Seung-Hyun Jan 25, 2024 05:49am
- For the first time, Samsung Biologics has surpassed a yearly operating profit of 1 trillion won among pharmaceutical companies in Korea. Due to the continued growth of CDMO services for biopharmaceuticals, the company has accumulated quarter sales exceeding 1 trillion won in consecutive quarters. According to the Financial Supervisory
- Company
- Sales of Esomezol ·Noltec↑ Nexium↓ in the PPI market
- by Kim, Jin-Gu Jan 25, 2024 05:49am
- Sales of major proton pump inhibitor (PPI) class of antiulcer drugs showed mixed performance last year. ‘Nexium (esomeprazole)’ saw a 4% year-on-year decline in prescriptions, while ‘Lanston LFDT (lansoprazole)’ and ‘Pariet (rabeprazole)’ also saw a decline in prescriptions. Hanmi Pharmaceutical's ‘Esomezol (esomeprazole)’ and Ilyan
- Company
- Potential of AVEO Oncology acquired by LG Chem
- by Jan 24, 2024 05:46am
- LG Chem's subsidiary AVEO Oncology is showing results in intractable cancers. AVEO, which was acquired by LG Chem in 2022 for about KRW 750 billion, specializes in developing new anticancer drugs and has recorded annual sales of about KRW 200 billion. AVEO owns Fotivda (tivozanib), which has been approved in the U.S. for the treatment of Stage I
- Company
- ‘I believe in MSD Korea and the Korean government'
- by Eo, Yun-Ho Jan 24, 2024 05:46am
- Applying for reimbursement extensions to 13 indications at the same time is an unprecedented move. This move was made by MSD Korea for its immuno-oncology drug Keytruda (pembrolizumab), and the case has been marked as a ‘historical first’ ever since the introduction of the positive-listing system in Korea. Applying for the reimbursement
- Company
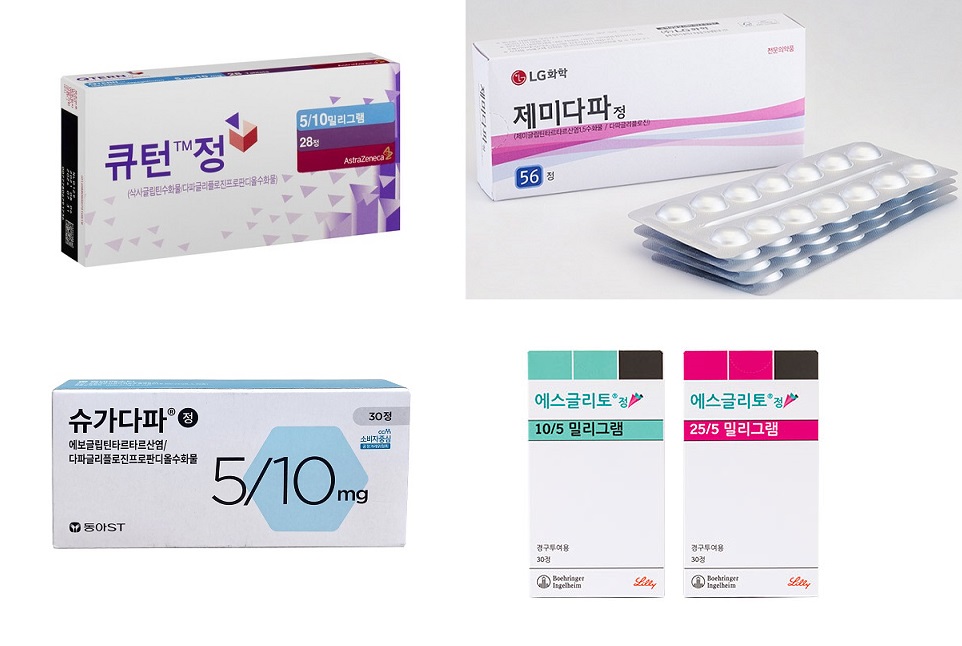
- SGLT2i+DPP4i combos' performance falls short of expectations
- by Kim, Jin-Gu Jan 23, 2024 06:02am
- A large number of 'SGLT-2 inhibitor (SGLT2i)+DPP-4 inhibitor (DPP4i)' combinations entered the market after Korea’s insurance reimbursement was extended to cover ‘SGLT-2 inhibitor + DPP-4 inhibitor’ combinations, however, their sales performance in the first year is falling short of expectations. Of the major products that have been on
- Company
- Will Verzenio secure expansion for early breast cancer?
- by Eo, Yun-Ho Jan 23, 2024 06:02am
- ‘Verzenio’, which faced difficulties in expanding its reimbursement standards last year, is now drawing attention to see if it will pass the review this time. According to industry sources, Lily Korea’s CDK4/6 inhibitor Verzenio (abemaciclib) is expected to be considered for the Health Insurance Review and Assessment Service (HIRA)
- Company
- Sotatercept receives orphan drug designation in Korea
- by Eo, Yun-Ho Jan 22, 2024 05:49am
- Sotatercept, a new drug candidate for pulmonary arterial hypertension (PAH), has been designated an orphan drug in Korea. The Ministry of Food and Drug Safety recently announced the drug’s designation in the first orphan drug designation announcement it made in the new year. Sotatercept, which is being developed by Merck, is being re
- Company
- First oHCM drug Camzyos applies for reimb in Korea
- by Eo, Yun-Ho Jan 22, 2024 05:49am
- The first-ever obstructive hypertrophic cardiomyopathy (oHCM) treatment, ‘Camzyos,’ is being reviewed for reimbursement in Korea. According to industry sources, BMS Korea has recently applied for the reimbursement listing of its new obstructive hypertrophic cardiomyopathy (oHCM) drug Camzyos (mavacamten). Therefore, whether the drug
- Company
- Hemlibra reduces risk of exercise-associated bleeding
- by Lee, Seok-Jun Jan 19, 2024 05:44am
- The research findings, which include data on the physical activities of patients treated with JW Pharmaceutical’s hemophilia A treatment ‘Hemlibra (emicizumab)’ and their safety profile, have been published in the International Journal of Hematology (Int J Hematol). Hemlibra is an innovative new drug that mimics the function of blood
- Company
- Keytruda's reimb for 6 indications will be reviewed
- by Eo, Yun-Ho Jan 19, 2024 05:44am
- The reimbursement challenge for Keytruda continues this year. Although no results have been made, the company seems to be on a steady course in applying for reimbursement. According to industry sources, 6 indications of MSD Korea’s PD-1 inhibitor immuno-oncology drug Keytruda (pembrolizumab) are expected to be submitted to the Health Ins