- Company
- AstraZeneca appoints Sewhan Chon as new Country President
- by Eo, Yun-Ho Dec 1, 2023 05:35am
- Sewhan Chon (49), former Country President of AstraZeneca Indonesia, has been appointed the new Country President of AstraZeneca Korea. According to industry sources, Sewhan Chon has officially taken office as the Country President of AstraZeneca Korea as of the 1st. With the appointment, the former Country President, Kim Sang-pyo (53),
- Company
- Demand for ADC Enhertu rise despite nonreimbursement
- by Kim, Jin-Gu Nov 30, 2023 05:55am
- Cumulative sales of Daiichi Sankyo’s HER2-positive breast cancer treatment ‘Enhertu (trastuzumab-deruxtecan)’ have exceeded KRW 10 billion even without reimbursement. According to the market research institution IQVIA on the 27th, Enhertu's cumulative sales were KRW 13.8 billion by Q3 this year. Sales steadily increased from KRW 2.2 bi
- Company
- Samsung Biologics’ CMO orders exceed KRW 3 trillion this yr
- by Chon, Seung-Hyun Nov 29, 2023 05:50am
- Samsung Biologics announced on the 28th that its contract manufacturing organization (CMO) orders this year will exceed KRW 3 trillion. On the day, the company publicly announced that it signed 1 new and 4 expanded CMO deals. Samsung Biologics signed a CMO deal worth KRW 588.8 billion with Asian pharmaceutical companies. In addition,
- Company
- Korean companies bid to break AZ’s monopoly with FcRN drug
- by Son, Hyung-Min Nov 28, 2023 05:42am
- With new FcRn antibody drugs secured by Korean pharmaceutical companies demonstrating efficacy in myasthenia gravis, these new FcRn antibody drugs are expected to rise as a competitor to C5 complement inhibitors such as AstraZeneca’s Ultromiris and Soliris that currently occupy the market in Korea.
- Company
- Budesonide’s price will be increased by up to 19%
- by Chon, Seung-Hyun Nov 28, 2023 05:41am
- The insurance ceiling price for asthma treatments containing ‘budesonide’ will be increased by up to 18.5%. The annual prescription market is expected to increase by more than KRW 1.5 billion. Once the supply and demand imbalance is resolved, the scope of market expansion is expected to increase further. According to the Ministry of Health
- Company
- Series of sales right transfers occur in KOR
- by Kim, Jin-Gu Nov 27, 2023 06:07am
- Competition between domestic pharmaceutical companies has been heating up for the rights to copromote vaccines by multinational pharmaceutical companies. With new products being released one after another in Korea, domestic companies are intent on becoming partners to secure these vaccines that generate stable cash flow. In fact, over
- Company
- CDK4/6 inhibitor mkt fluctuates with the rise of Kisqali
- by Son, Hyung-Min Nov 27, 2023 06:06am
- The CDK4/6 inhibitor market for breast cancer has changed from a monopoly to a duopoly in Korea. Kisqali, which had been introduced later into the market, has nearly caught up with Ibrance's sales. According to the market research institution IQVIA on the 24th, sales of Novartis’s Kisqali had risen 95.2% YoY to reach KRW 12.1 billion in Q3 t
- Company
- New AD drug Adtralza enters last stage to reimb in KOR
- by Eo, Yun-Ho Nov 27, 2023 06:06am
- The atopic dermatitis treatment ‘Adtralza’ is entering its final stages of receiving reimbursement in Korea. According to industry sources, the Ministry of Health and Welfare recently issued a negotiation order for Leo Pharma Korea’s interleukin-13 (IL-13) inhibitor for atopic dermatitis, Adtralza (tralokinumab). Under the order, the c
- Company
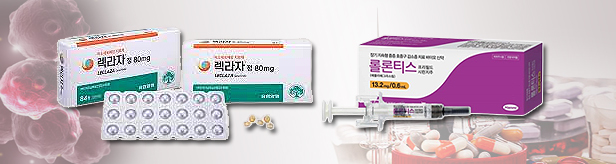
- Sales of K-anticancer drugs Leclaz and Rolontis rise in KOR
- by Chon, Seung-Hyun Nov 24, 2023 05:37am
- New anticancer drugs developed by domestic companies are gradually increasing their influence in their home market. Sales of Yuhan Corp’s lung cancer treatment Leclaza are expected to exceed KRW 20 billion this year. Also, cumulative sales of Hanmi Pharmaceutical's neutropenia treatment Rolontis have exceeded KRW 10 billion just 2 two into its
- Company
- MSD vaccines will be sold by Boryung·Kwangdong
- by Kim, Jin-Gu Nov 24, 2023 05:37am
- The domestic sales rights for 8 types of MSD vaccines have changed hands recently. Although HK Inno.N was in charge of joint sales for MSD vaccines in Korea, the vaccines will be separately sold by Boryung Biopharma and Kwangdong Pharmaceutical each. After signing a copromotion agreement with MSD for its vaccines at the end of 2021, HK