- Company
- Global 1 trillion won project
- by Kim, Jin-Gu Oct 19, 2023 05:29am
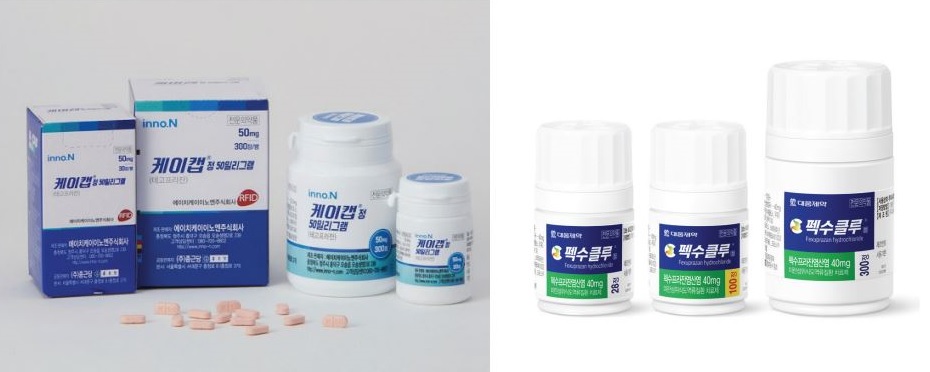
- Domestically developed new drugs in the P-CAB (Potassium-competitive gastric acid secretion inhibitor) series are accelerating their overseas expansion. HK inno.N K-CAB and Daewoong Pharmaceutical Fexuclue both set a global sales goal of 1 trillion won within five years. To this end, K-CAB has obtained product approval in 7 countries. In add
- Company
- Drug exports reduced by 14%... COVID-19 effect vanished
- by Kim, Jin-Gu Oct 18, 2023 05:48am
- Cumulative pharmaceutical exports in Q3 this year have fallen 13.8% YoY to a record KRW 5.8 trillion. With the global transition to a COVID-19 endemic, exports of COVID-19 vaccines fell sharply, leading to a decline in exports. According to the Korea Customs Service on the 17th, the cumulative pharmaceutical exports in Q3 this year amounted t
- Company
- Controversy arises over illegal rebates made by Company A
- by Nho, Byung Chul Oct 17, 2023 05:28am
- The multinational pharmaceutical company A's illegal rebate activities have gone too far and are disrupting competition in relevant markets. According to an anonymous tip on the 15th, Company A has been providing economic benefits to prescribers at large general hospitals to promote drug sales. The same company was investigated by th
- Company
- 'Verquvo is effective in high-risk heart failure patients'
- by Son, Hyung-Min Oct 17, 2023 05:28am
- Bayer’s new heart failure drug Verquvo (vericiguat) has been granted reimbursement listing in Korea, showing effect in high-risk patients. Based on the positive clinical results, experts have been claiming that Verquvo should be considered as a second-line treatment option. On the 16th, Bayer Korea held a press conference celebrating t
- Company
- Xtandi closes coinsurance gap with Erleada
- by Eo, Yun-Ho Oct 17, 2023 05:27am
- 'Xtandi' has virtually overcome its difference in drug price with ‘Erleada.’ Dailpharm’s coverage found out that Astellas Pharma Korea recently reached a final agreement with the National Health Insurance Service to negotiate the drug price for its prostate cancer treatment drug Xtandi (enzalutamide) to convert its indication for metas
- Company
- Kwangdong and Moderna strengthen partnership
- by Nho, Byung Chul Oct 16, 2023 05:24am
- Kwangdong Pharmaceutical (CEO Choi Seong-won) announced on the 13th that it will begin full-scale activities to provide medical information to medical staff about Moderna's newly updated COVID-19 vaccine 'Spikevax X', which was recently approved by the Ministry of Food and Drug Safety. Moderna's monovalent vaccine against the XBB.1.5 mutat
- Company
- Lioresal Intrathecal can be prescribed in general hospitals
- by Eo, Yun-Ho Oct 16, 2023 05:24am
- The skeletal muscle relaxant and antispastic ‘Lioresal Intrathecal (baclofen injection)’ may now be prescribed in general hospitals in Korea According to industry sources, Novartis' Lioresal (baclofen), which will be supplied through the Korea Orphan & Essential Drug Center, has passed the Drug Committees (DCs) of tertiary hospitals inc
- Company
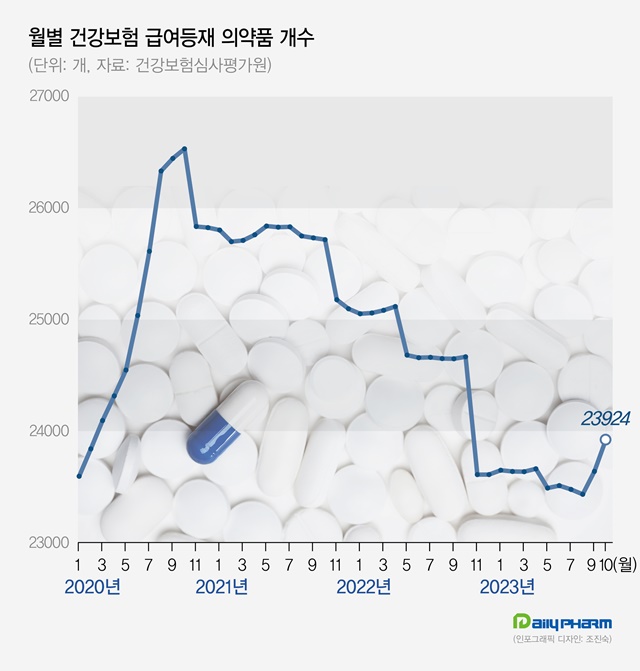
- Record number of reimbursed drugs listed in 1 yr
- by Chon, Seung-Hyun Oct 16, 2023 05:24am
- The number of drugs listed for health insurance reimbursement reached a record high in one year. After the generic market for the diabetes treatment drug ‘Januvia’ opened up, the number of reimbursed drugs expanded rapidly. With single-agent and combination-drug generics of Januvia that were approved under consignment contracts signed before t
- Company
- Will Paxlovid open a market for elderly people’s benefits?
- by Eo, Yun-Ho Oct 16, 2023 05:24am
- As the sense of crisis regarding COVID-19 infection is decreasing, attention is being paid to whether Paxlovid, a treatment drug, can successfully be registered for insurance benefits. Pfizer Korea is currently proceeding with the registration process for Paxlovid, which has been approved as an oral treatment for COVID-19, with the goal of l
- Company
- Bayer Korea appoints JinA Lee as new Managing Director
- by Eo, Yun-Ho Oct 13, 2023 05:29am
- Bayer Korea appointed JinA Lee (54) as the new Managing Director. With this appointment, Lee will become the first Korean head to be appointed to the establishment of Bayer Korea. The company recently announced through an internal announcement that it will appoint JinA Lee, former CDH (Country Division Head) of Pharmaceuticals and Managin