- Company
- Hanmi will focus on anti-obesity drugs... from GLP-1 to DTx
- by Kim, Jin-Gu Sep 14, 2023 06:37am
- Hanmi Science has pointed to obesity management as a future growth engine for the Hanmi Group. Hanmi Science plans to select 5 types of pipelines, including a new GLP-1 obesity treatment, and operate the project under the name, 'H.O.P (Hanmi Obesity Pipeline)'. The H.O.P project includes 5 types of treatment that include ▲'efpeglenatid
- Company
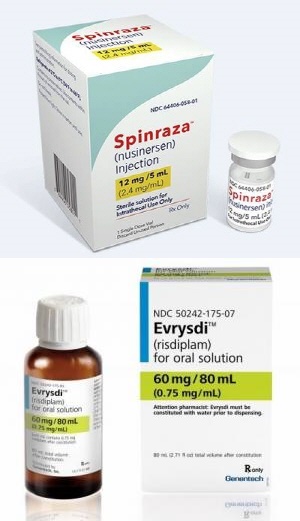
- SMA drugs Spinraza and Evrysdi complete pricing negotiations
- by Eo, Yun-Ho Sep 14, 2023 06:36am
- The spinal muscular atrophy (SMA) treatment ‘Evrysdi (risdiplam)’ finally passed the final hurdle to its reimbursement in Korea According to industry sources, both SMA treatments, Biogen Korea’s ‘Spinraza (nusinersen)’ and Roche Korea’s treatment ‘Evrysdi (risdiplam),’ reached a final agreement with the National Health Insurance S
- Company
- Obesity is a chronic disease
- by Kim, Jin-Gu Sep 12, 2023 05:37am
- “Currently, only obesity metabolic surgery is covered. The scope needs to be expanded to include obesity treatments, etc.” “Obesity is a chronic disease like high blood pressure and diabetes. We need to apply health insurance benefits from a treatment perspective, not from a beauty perspective.” Kim Gyeong-gon, vice president of the K
- Company
- Celltrion’s Remsima celebrates 10th year of export
- by Chon, Seung-Hyun Sep 12, 2023 05:37am
- Celltrion’s first biosimilar, ‘Remsima’, has marked its 10th anniversary in entering the overseas market. Together with its subcutaneous injection formulation Remsima SC, Remsima successfully settled in the European market and recorded total exports that exceeded KRW 6 trillion over the past 10 years. &8232; Celltrion Healthcare announced
- Company
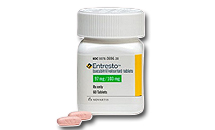
- Entresto’s 2nd patent suit ruling postponed to November
- by Kim, Jin-Gu Sep 12, 2023 05:37am
- The rulings for the 2nd trial surrounding the chronic heart failure treatment ‘Entresto (sacubitril/valsartan)’ have been postponed to November 9. The drug has been posting&160;annual prescriptions of more than KRW 40 billion annually. &8232; According to industry sources on the 11th, the Patent Court of Korea 1st Division recently pos
- Company
- Dinutuximab beta was designated as an orphan drug
- by Eo, Yun-Ho Sep 12, 2023 05:37am
- Dinutuximab beta, an immunotherapy drug administered to neuroblastoma patients, has been designated as an orphan drug. Dinutuximab is a drug from EUSA Pharma, a domestic corporation launched last year, and is currently undergoing formal approval procedures. EUSA Pharma was absorbed and merged into Recordati in 2021. This drug is a chimeric
- Company
- Different progress of three new asthma drugs launched
- by Eo, Yun-Ho Sep 11, 2023 05:29am
- The results of the three types of asthma antibody drugs were different. According to the related industry, at the HIRA Pharmaceutical Reimbursement Evaluation Committee held on the 7th, Nucala of GSK Korea was judged to be appropriate for reimbursement, and Fasenra of AstraZeneca Korea was judged to be non-reimbursable. These drugs are in
- Company
- Rebamipide survives reimb evaluations...a relief
- by Chon, Seung-Hyun Sep 11, 2023 05:29am
- The gastric ulcer treatment ‘rebamipide’ survived Korea’s reimbursement reevaluations. As a result, pharmaceutical companies were able to hold on to their cash cow that brings in KRW 140 billion per year. The Health Insurance Review and Assessment Service concluded that the active substance rebamipide was adequate for reimbursement after d
- Company
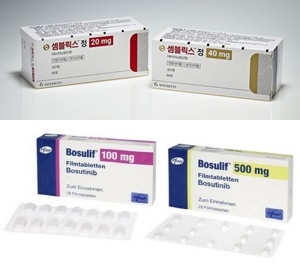
- 2nd-gen CML drug approved for reimb following 4th-gen drug
- by Eo, Yun-Ho Sep 11, 2023 05:29am
- Companies are actively working to expand insurance coverage for their prescription chronic myeloid leukemia treatments in Korea. According to the industry sources, Novartis Korea's 4th generation CML treatment ‘Scemblix (asciminib)' was listed for reimbursement last July, and Pfizer Korea's 2nd generation drug 'Bosulif (bosutinib)' also
- Company
- The generic for exclusivity period for Vemlidy ends
- by Kim, Jin-Gu Sep 8, 2023 05:34am
- The generic exclusivity period for the hepatitis B treatment Vemlidy expires on the 15th of this month. Five additional companies that have received approval for generic drugs for this ingredient are expected to enter the market. The pharmaceutical industry is paying attention to whether the original will have an impact on the market share,