- Company
- BsAb lymphoma treatment Lunsumio will soon land in KOR
- by Eo, Yun-Ho Jul 17, 2023 05:30am
- The new bispecific antibody treatment for lymphoma, ‘Lunsumio’, will soon land in Korea. According to industry sources, the Ministry of Food and Drug Safety is in its last stages of review for the product approval of Roche Korea's follicular lymphoma drug Lunsumio (mosunetuzumab), which was designated as the first drug subject to Korea
- Company
- Danicopan has been designated for GIFT
- by Eo, Yun-Ho Jul 17, 2023 05:30am
- Danicopan, a combination partner of Soliris, was designated as a GIFT at the same time as this orphan drug designation. The Ministry of Food and Drug Safety recently announced that AstraZeneca's Danicopan (ALXN2040) will be designated as an orphan drug and GIFT in Korea. Danicopan, a candidate for the treatment of paroxysmal nocturnal hem
- Company
- "Cibinqo secures solid data in atopic dermatitis"
- by Jung, Sae-Im Jul 13, 2023 05:35am
- 'Cibinqo (abrocitinib),' the third JAK inhibitor to be introduced for atopic dermatitis in Korea, will be reimbursed starting this month. Healthcare professionals have expressed high expectations for Cibinqo, as the drug has accumulated robust data in treating severe atopic dermatitis. On the 11th, the Korean pharmaceutical company Hanmi Phar
- Company
- Schizophrenia drug Invega Hafyera lands in general hospitals
- by Eo, Yun-Ho Jul 13, 2023 05:35am
- The long-acting formulation of the schizophrenia drug ‘Invega’ has landed in general hospitals in Korea. According to industry sources, Invega Hafyera 1092mg/1560mg (paliperidone palmitate), Janssen Korea’s extended-release injectable suspension for patients with schizophrenia that is injected every 6 months, has passed the drug commit
- Company
- Samsung Biologics, cumulative order amt exceeded 2 trillion
- by Hwang, Jin-joon Jul 11, 2023 05:41am
- Samsung Biologics announced on the 10th that it has signed a main contract for biopharmaceutical consignment manufacturing (CMO) worth 511.1 billion won from global pharmaceutical company Novartis. Through this contract, the cumulative amount of orders received this year increased to 2.3387 trillion won. This contract with Novartis was signed
- Company
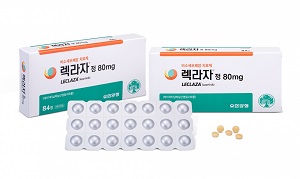
- ‘Leclaza will be free as first-line Tx until reimbursement'
- by Kim, Jin-Gu Jul 11, 2023 05:41am
- Wook-Je Cho, CEO of Yuhan Corp announced that it will be providing its anticancer drug ‘Leclaza (lasertinib)’ for free as a first-line treatment until its reimbursement is extended to cover first-line treatment. Cho announced the company’s launch of the Early Access Program (EAP) at a press conference it had held on the 10th at the Seo
- Company
- 'Leclaza effective as 1st-line treatment in Koreans'
- by Kim, Jin-Gu Jul 11, 2023 05:41am
- On the morning of the 10th, Professor Jin-Hyoung Kang of Medical Oncology at the Catholic University of Korea Seoul St. Mary's Hospital, said, "LASER 301,' the global Phase III clinical trial on Leclaza (lazertinib), is the only study that demonstrated the drug's efficacy as a first-line treatment for EGFR mutation-positive non-small cell
- Company
- Pricing negotiations for Spinraza's reimb begin in KOR
- by Eo, Yun-Ho Jul 11, 2023 05:41am
- The spinal muscular atrophy treatment ‘Spinraza’ has finally entered the last stage of its reimbursement extension in Korea. According to Dailypharm coverage, Biogen is undergoing drug pricing negotiations with the National Health Insurance Service for its SMA treatment Spinraza (nusinersen). Also, another SMA treatment, Roche Korea
- Company
- Yuhan provides Leclaza with 6 million won per month for free
- by Kim, Jin-Gu Jul 11, 2023 05:40am
- Regarding Yuhan's providing free medicines to patients until the first treatment of Leclaza is covered by insurance, Yuhan said, "It is not an act of inducing patients and there is no problem under the Fair Trade Act." On the 10th, Yuhan held a press conference for permission for the first treatment of Leclaza at the Plaza Hotel in Seoul.
- Company
- Will the non-reimbursed Gavreto vanish in Korea?
- by Jung, Sae-Im Jul 10, 2023 05:21am
- The possibility that the new RET-targeted anticancer therapy ‘Gavreto (pralsetinib)’ will vanish without ever being released in Korea has been rising. With Roche giving up sales rights for Gavreto, the prospects of its reimbursement listing in Korea had also gone up in smoke. As Blueprint Medicines, the original developer of Gavreto, does