- Company
- 3 new asthma drugs resumed discussion on reimbursement
- by Eo, Yun-Ho Jul 10, 2023 05:21am
- Three new asthma drugs, which had been uninsured for at least three years, started the insurance benefit listing process, but only one drug made progress. According to related industries, Handokteva's eosinophilic asthma treatment Cinqair passed the HIRA Pharmaceutical Reimbursement Evaluation Committee. However, GSK Korea's Nucala and Ko
- Company
- New AML drug Vyxeos can be prescribed at general hospitals
- by Eo, Yun-Ho Jul 10, 2023 05:21am
- The new drug ‘Vyxeos’ that was introduced to Korea by Handok Pharmaceuticals may now be prescribed at general hospitals in Korea, According to industry sources, Vyxeos (daunorubicin+ cytarabine), a treatment for adults with newly diagnosed, therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-
- Company
- Daiichi's sales rose & Astellas' sales fell
- by Jung, Sae-Im Jul 10, 2023 05:20am
- Among Japanese pharmaceutical companies that have entered the domestic market, 4 out of 7 subsidiaries with settlements in March expanded their sales. Daiichi Sankyo Korea's sales reached 253.2 billion won, ranking first among Japanese pharmaceutical companies. As a result of analyzing the performance for the 2022 business year (April 1, 2
- Company
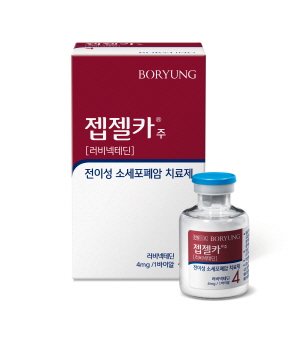
- Boryung’s new drug Zepzelca lands in Big 5 hospitals in KOR
- by Eo, Yun-Ho Jul 7, 2023 05:43am
- Zepzelca, a new drug introduced to Korea by Boryung, can now be prescribed in the Big 5 general hospitals in Korea. According to industry sources, the small cell lung cancer treatment Zepzelca (lurbinectedin) has passed the drug committees (DCs) of all the top 5 tertiary hospitals in Korea &8211; Samsung Medical Center, Seoul National U
- Company
- Release of triple-combo diabetes drugs imminent
- by Kim, Jin-Gu Jul 7, 2023 05:43am
- The release of the ‘triple combination therapies' appear to be in sight. However, unlike the 2-drug combos that are already in fierce competition even in the pre-release stage, industry interest in 3-drug combos is currently at best lukewarm. The pharmaceutical industry pointed to the relatively challenging development environment for
- Company
- Baxter and Fresenius Medical benefited the most
- by Nho, Byung Chul Jul 7, 2023 05:43am
- As the Ministry of Health and Welfare's pilot project for home management of peritoneal dialysis patients began in earnest, the sales of Baxter and Fresenius Medical, which supply related products, are also expected to grow exponentially. As of 2022, total domestic hemodialysis patient medical expenses amounted to 2,634 billion won, of which
- Company
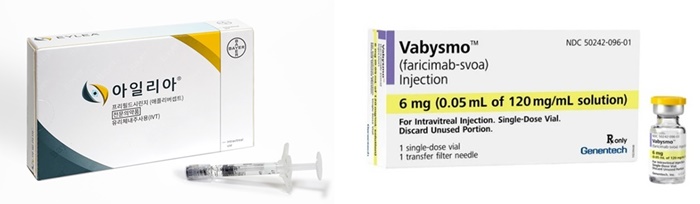
- Vabysmo’s real-world data to hold leader Eylea in check
- by Jung, Sae-Im Jul 6, 2023 05:39am
- The competition between the current lead ‘Eylea’ and the new drug ‘Vabysmo’ is fierce in the macular degeneration treatment market. Based on the real-world data that demonstrated Vabysmo’s consistent effect on patients who switched from Eylea, the company built evidence for patients to switch to Vabysmo. To defend the lead, Eylea’s co
- Company
- Industry anxiously awaits reimb of SGLT-2 inhibitors
- by Jung, Sae-Im Jul 6, 2023 05:39am
- The reimbursement applications that companies of SGLT-2 inhibitors filed to receive reimbursement for their heart failure indication are expected to undergo a difficult journey. The largest barrier to their reimbursement is the concern over the enormous amount of fiscal spending the drugs will bring due to the broad target patient group.
- Company
- Sidapvia, a combination of Forxiga in collaboration
- by Jung, Sae-Im Jul 6, 2023 05:38am
- AstraZeneca Korea announced on the 5th that it has obtained permission from the Ministry of Food and Drug Safety for the Forxiga combination 'Sidapvia (Dapagliflozin 10mg + Sitagliptin 100mg)'. Sidapvia, approved as a treatment for type 2 diabetes, is a combination drug that combines the SGLT-2 inhibitor Dapagliflozin's original product Forx
- Company
- Dongkook and GC agree to co-promote Glarzia in Korea
- by Kim, Jin-Gu Jul 5, 2023 05:45am
- On the 3rd, Dongkook Pharmaceutical and GC Biopharma announced that the two companies have signed a business agreement for the domestic sales and marketing of ‘Glarzia Prefilled Pen (insulin glargine),’ a biosimilar of the insulin injection Lantus. Glarzia is a Lantus biosimilar developed by the Indian biosimilar pharmaceutical company