- Company
- The efficient use of CDK 4/6 needs to be discussed
- by Jun 14, 2023 05:38am
- CDK4/6 inhibitors, which were prevalent in metastatic breast cancer, have expanded their scope to early breast cancer. Following Lily Verzenio, Novartis Kisqali demonstrated the effect of adjuvant therapy after surgery through a clinical presentation this year. As the role of CDK4/6 inhibitors expands, new concerns are emerging to achieve the
- Company
- Hanmi to examine the possibility of the Poseltinib effect
- by Chon, Seung-Hyun Jun 14, 2023 05:38am
- Hanmi Pharm is examining the possibility of new indications with new drug candidates returned by multinational pharmaceutical company Eli Lilly. Hanmi Pharmaceutical announced on the 12th that it announced the interim results of phase 2 clinical trial of three-drug combination therapy, a follow-up study of the BTK inhibitor Poseltinib, at th
- Company
- Hanmi's NASH drug candidate, designated as a fast track
- by Jun 14, 2023 05:38am
- Hanmi Pharmaceutical announced on the 13th that Efinopegdutide, a new drug candidate for non-alcoholic steatohepatitis (NASH), has been designated as fast-track by the US Food and Drug Administration (FDA). Efinopegdutide is a dual agonist that simultaneously activates the GLP-1 receptor, which helps the secretion of insulin and suppresses ap
- Company
- Will a new treatment option be introduced for gastric cancer
- by Jung, Sae-Im Jun 14, 2023 05:38am
- Unlike lung cancer and breast cancer, gastric cancer has been regarded as one type of cancer that has not benefited from the development of new anticancer drugs. For the past decade, chemotherapy has been the standard first-line treatment for patients with HER2 gene-negative metastatic gastric cancer. This means that there were no suitable
- Company
- Rozanolixizumab has been designated as an orphan drug
- by Eo, Yun-Ho Jun 14, 2023 05:37am
- Rozanolixizumab, a new drug for generalized myasthenia gravis, has been designated as an orphan drug. The Ministry of Food and Drug Safety recently announced that it designated UCB's gMG treatment Rozanolixizumab as an orphan drug. The target indication is the treatment of generalized myasthenia gravis in patients who are anti-acetylcholin
- Company
- Ildong makes initial lead in the SGLT2·DPP4 combo market
- by Kim, Jin-Gu Jun 14, 2023 05:35am
- Full-fledged competition has begun in the ‘SGLT-2 inhibitor+DPP-4 inhibitor’ two-drug combination therapy market for the treatment of diabetes. In the first month after 5 products were launched, outpatient prescriptions reached KRW 300 million, and AstraZeneca and Ildong Pharmaceutical's Qtern led the market bringing in prescriptions of
- Company
- Discussions on expanding benefit for Jakavi's GVHD are slow
- by Eo, Yun-Ho Jun 13, 2023 05:43am
- Discussions on expanding insurance coverage for Jakavi's Graft-versus-Host Disease (GvHD) indication have been sluggish. According to the industry, Novartis Korea Jakavi passed the harmaceutical Reimbursement Criteria Subcommittee of the Health Insurance Review and Assessment Service last February, but it has not been submitted to the Financi
- Company
- Severe asthma Tx Tezpire to soon be commercialized in Korea
- by Eo, Yun-Ho Jun 13, 2023 05:43am
- The new drug for severe asthma, ‘Tezpire' is soon to enter the Korean market. According to industry sources, AstraZeneca Korea has submitted an application for the approval of ‘Tezspire (tezepelumab)’ in Q2 to the Ministry of Food and Drug Safety and is receiving review. At this pace, Tezspire is expected to be commercialized in the se
- Company
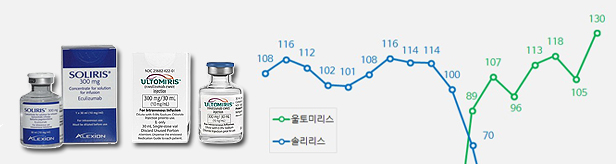
- Sales of Soliris and Ultomiris rise despite changes
- by Kim, Jin-Gu Jun 13, 2023 05:43am
- Quarterly sales of the rare disease treatments ‘Soliris (eculizumab)’ and ‘Ultomiris (ravulizumab)’ had risen significantly in Q1 this year. Although sales of Soliris remained similar to last year, sales of Ultomiris, its follow-on drug, soared in the same period. The analysis is that the generation change between the two drugs has neare
- Company
- Beova can be prescribed at general hospitals
- by Eo, Yun-Ho Jun 12, 2023 05:42am
- Beova, a new drug introduced by Jeil Pharmaceutical, will be available for Rx at general hospitals. According to related industries, Beova, an overactive bladder treatment, is currently available at 22 medical institutions nationwide, including SMC and AMC, as well as advanced general hospitals, Gangnam Severance Hospital, Sangnam Sacred Hear