- Company
- Pharma industry on alert over business risks in China
- by Lee, Seok-Jun May 24, 2023 05:32am
- Business risks related to China have been rising in Korea’s pharmaceutical industry. There are many causes, including the termination of the contract for supplying medicines (or cosmetics), liquidation of Chinese subsidiaries, and claims for damages, etc. Most of them are due to the failure of their Chinese partners in fulfilling their promises
- Company
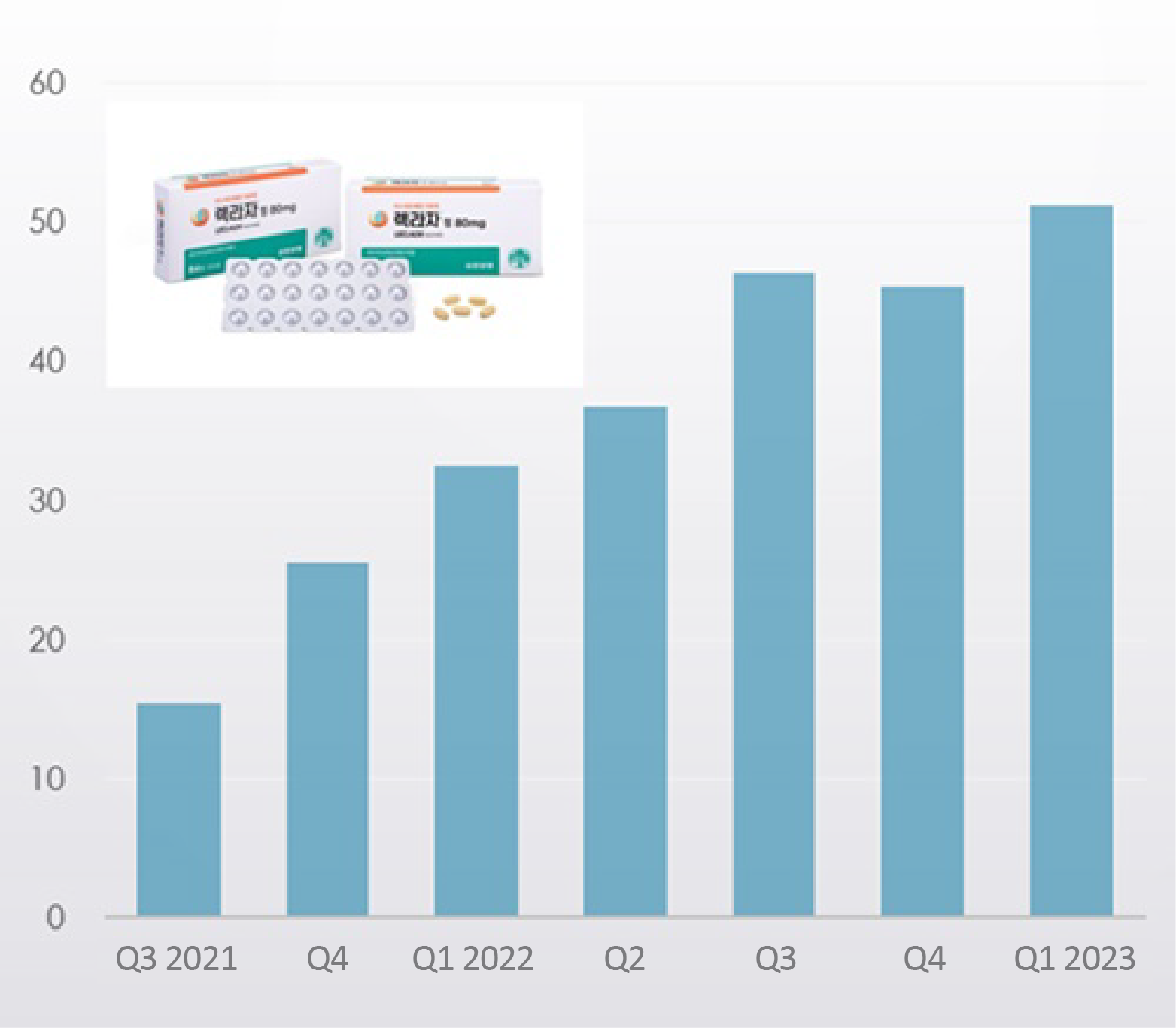
- Leclaza posts sales of KRW 25 bil in 2 years in Korea
- by Chon, Seung-Hyun May 24, 2023 05:32am
- Yuhan Corp’s anticancer drug ‘Leclaza’ is making good sales in the Korean market, and raised sales of KRW 5.1 billion in Q1 alone. Its efficacy and safety were confirmed in the real world in actual patients at the time of treatment, and the drug is gradually expanding its market influence ahead of its approval as a first-line treatment. Ac
- Company
- Merck retrieves rights to PD-L1 antibody Bavencio in Korea
- by Eo, Yun-Ho May 23, 2023 05:54am
- The long-standing collaboration that had existed between the Korean subsidiaries of Merck and Pfizer Korea for the immunotherapy ‘Bavencio’ has come to a close. According to industry sources, the companies are in the process of handling the rights for the PD-L1-inhibiting immunotherapy Bavencio (avelumab) in Korea as Merck retrieved th
- Company
- RWD results reaffirm Leclaza’s efficacy in practice
- by Chon, Seung-Hyun May 23, 2023 05:54am
- Study results that confirm the efficacy and safety of Yuhan Corp's new anticancer drug ‘Leclaza’ in the real world has been released. Lim Sun Min, Professor of Oncology at Yonsei Cancer Center, and Beung-Chul Ahn, Professor of Oncology at the National Cancer Center, met with reporters at the Korea Pharmaceutical and Bio-Pharma Manufact
- Company
- SGLT2 lowers BP, but more evidence is needed to use it alone
- by Hwang, Jin-joon May 23, 2023 05:50am
- There was an opinion that there is still insufficient evidence for the use of the sodium-glucose cotransporter-2 (SGLT-2) inhibitor, a treatment for type 2 diabetes and heart failure, for the treatment of hypertension. It can be expected to lower blood pressure in patients with heart failure or diabetes, but it is difficult to use it alone f
- Company
- AZ runs a neurofibromatosis disease awareness campaign
- by Jung, Sae-Im May 22, 2023 05:42am
- AstraZeneca Korea announced on the 18th that it had conducted the 'Twinkling a Light for NF-1 Campaign' for its executives and employees to support domestic neurofibromatosis patients in celebration of 'World Neurofibromatosis Awareness Day'. The Children's Cancer Foundation designated May 17 every year as World Neurofibromatosis Awareness Day t
- Company
- Hana Pharm, signed a sub-license agreement for Byfav
- by Lee, Seok-Jun May 22, 2023 05:42am
- Hana Pharm announced on the 18th that it had signed a sub-license contract with Hyphens Pharma of Singapore for the exclusive rights to Byfavo 20mg, an anesthetic new drug. This contract is the first achievement of local partnering while Hana Pharm received licenses for six Southeast Asian countries from German PiON in 2020 and was in the pro
- Company
- New formulations for schizophrenia are being commercialized
- by Eo, Yun-Ho May 22, 2023 05:42am
- According to related industries, new long-acting formulations of existing schizophrenia treatment drugs, such as Abilify and Invega, are being released one after another. Lundbeck and Otsuka Pharmaceutical obtained US FDA approval for Abilify Asimtufii, which is administered once every two months, last month. Abilify Asimtufii confirmed i
- Company
- “Need to increase support for new AI drugs in Korea”
- by Jung, Sae-Im May 22, 2023 05:42am
- “Although artificial intelligence (AI) new drug development ecosystem is being created in Korea, there are still many areas that we are lacking at a global level in terms of manpower or investment scale. Chinese companies that were established around the same time received more than KRW 500 billion in investments, while Korean companies only re
- Company
- Attempts to develop new drugs for hypertension
- by Hwang, Jin-joon May 22, 2023 05:41am
- Opinions were raised that it would be difficult for candidates under development as new drugs for hypertension, such as Baxdrostat, Aprocitentan, and Firibastat, to replace existing drugs. It is expected that it will fill the unmet demand rather than take the place of the prescribed treatment. Professor Woong-Gil Choi of Chungbuk National