- Company
- Crysvita, the first fast-track drug by Yoon gov, is expected
- by Eo, Yun-Ho May 16, 2023 09:06pm
- It is now possible to prescribe 'Crysvita', the No. 1 rapidly registered drug of the Yoon government. This drug recently passed the Drug Committee (DC) of medical institutions such as SNUH. Kyowa Kirin Korea's (XLH rickets treatment Crysvita is a pediatric treatment that has been clinically proven effective in the field of diseases for whi
- Company
- JAKi Cibinqo owns strengths in dosage adjustments
- by Jung, Sae-Im May 16, 2023 05:40am
- Pfizer's JAK inhibitor ‘Cibinqo (abrocitinib)' has embarked on a full-fledged journey to expand its prescriptions. After landing in major general hospitals at the end of last year, the drug is likely to be registered for reimbursement within the first half of this year. Cibinqo is a Janus kinase 1 (JAK1) inhibitor approved by the Ministry
- Company
- Samil, a series of love calls from multinational companies
- by Lee, Seok-Jun May 16, 2023 12:16am
- Samil Pharmaceutical is receiving a series of love calls from multinational companies. This time, it has decided to exclusively distribute and sell all Sandoz products, including the central nervous system (CNS). The CNS division, which was newly established in 2021, will gain momentum for business expansion. It is evaluated that Samil Pharma
- Company
- New diabetes treatment guidelines released
- by Kim, Jin-Gu May 15, 2023 05:41am
- The status of GLP-1 analogues and SGLT-2 inhibitors has risen further in Korea’s new diabetes treatment guidelines. In the revised guidelines, when considering options to use in combination with injection therapy, GLP-1 analogues were recommended over basal insulin, and SGLT-2 inhibitors were recommended first for diabetic patients with hear
- Company
- MSD Korea begins voluntary retirement
- by Jung, Sae-Im May 15, 2023 05:40am
- MSD Korea, which announced a reduction in manpower due to the abolition of the Januvia division, has begun a full-fledged reduction in personnel by disclosing the conditions for ERP. The labor union of MSD Korea warned of a tough response, saying, "We cannot accept the company's attempt to reduce manpower." On the 12th, MSD Korea announced t
- Company
- Advate leading hemophilia treatment
- by Nho, Byung Chul May 12, 2023 05:45am
- In the field of hemophilia non-antibody and antibody treatment, Takeda Korea Pharmaceutical and JW Pharmaceutical are gaining attention as they are strengthening their positions. Korea Takeda Pharmaceutical's 'Advate/Adynovate', and JW Pharmaceutical's Hemlibra's sales last year were 26.2 billion won and 7.6 billion won, respectively, ran
- Company
- SK Biopharm’s cenobamate secures KRW 700 billion
- by Chon, Seung-Hyun May 12, 2023 05:45am
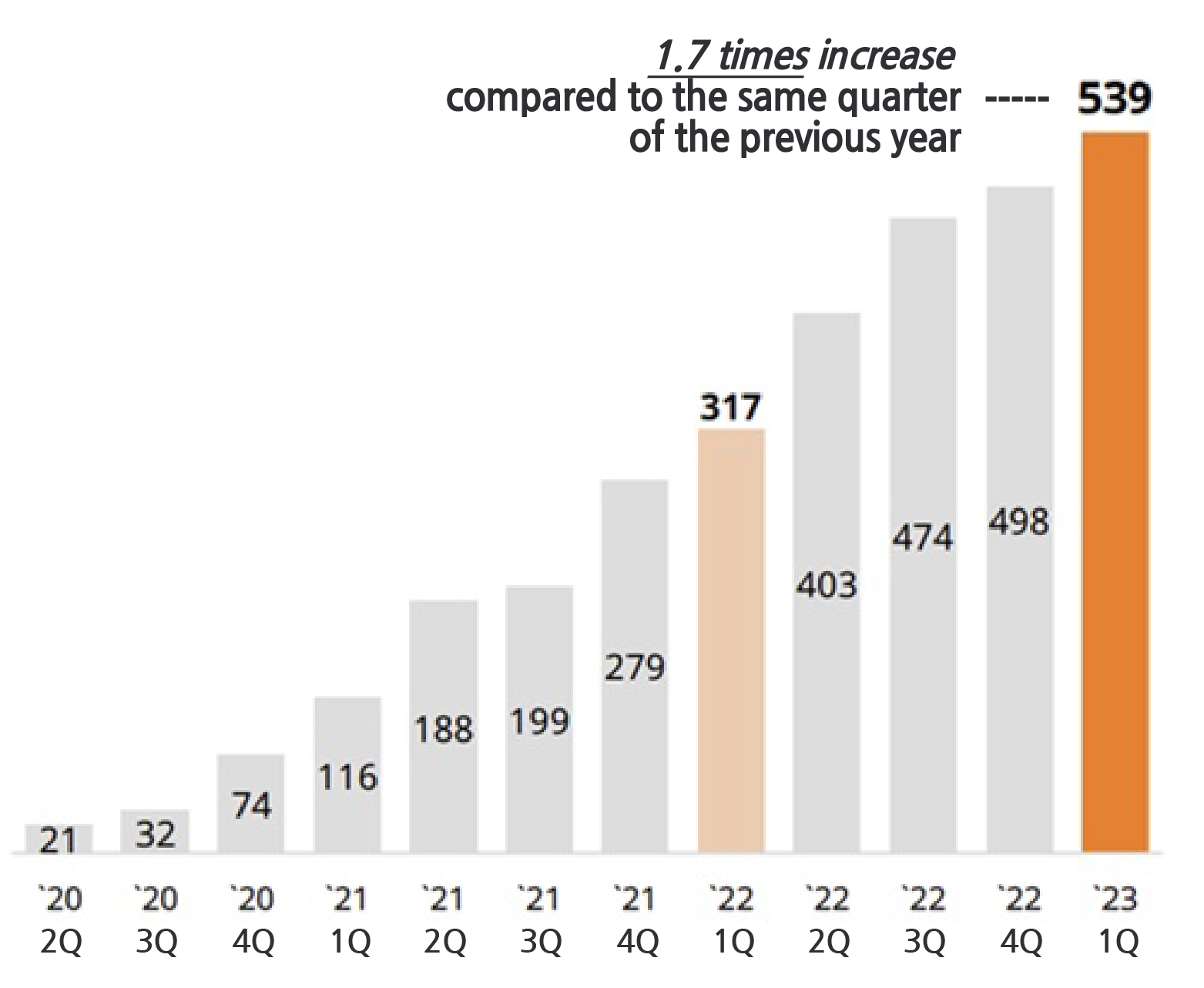
- Cumulative sales of SK Biopharmaceutical’s epilepsy treatment cenobamate exceeded KRW 300 billion in the US. It had continued to show growth every quarter ever since its release. Combined with the upfront payment and milestone payments cenobamate has earned more than KRW 700 billion over the past 4 years. According to SK Biopharm on the 11th
- Company
- SU's strengths maximized through combined use with SGLT-2is
- by Kim, Jin-Gu May 12, 2023 05:44am
- New opportunities have emerged for the use of the diabetes treatment sulfonylurea (SU). It is claimed that its combined use with SGLT-2 inhibitor drugs can offset the existing disadvantages such as hypoglycemia and weight gain, while fully utilizing the strong blood sugar lowering effect of SU drugs. Professor Jin Hwa Kim of the Depart
- Company
- Scemblix can be prescribed at general hospitals
- by Eo, Yun-Ho May 11, 2023 05:48am
- Novartis' new chronic myelogenous leukemia drug Scemblix is entering prescription rights in general hospitals. According to related industries, Novartis Korea's Ph+CML Philadelphia chromosome-positive chronic myeloid leukemia treatment drug Scemblix has been approved by the Drug Committee (DC) of medical institutions such as Seoul St. Mary
- Company
- Scemblix can be prescribed at general hospitals
- by Eo, Yun-Ho May 10, 2023 11:18pm
- Novartis' new chronic myelogenous leukemia drug Scemblix is entering prescription rights in general hospitals. According to related industries, Novartis Korea's Ph+CML Philadelphia chromosome-positive chronic myeloid leukemia treatment drug Scemblix has been approved by the Drug Committee (DC) of medical institutions such as Seoul St. Mar