- Company
- US approval of Celltrion Yuflyma was delayed
- by Feb 24, 2023 05:53am
- Celltrion has confirmed that the U.S. Food and Drug Administration (FDA) will complete the Yuflyma final approval review by May of this year. Celltrion announced on the 23rd that it had confirmed that the final approval review of Yuflyma would be completed by May of this year while continuing discussions with the FDA. Earlier, the U.S. approv
- Company
- Shingrix may be prescribed in 93 general hospitals in Korea
- by Eo, Yun-Ho Feb 24, 2023 05:52am
- The shingles vaccine ‘Shingrix’ has quickly landed in medical institutions in Korea after starting vaccinations. According to industry sources, GSK Korea’s recombinant vaccine Shingrix passed review by drug committees of 93 medical institutions in Korea, including tertiary hospitals - Samsung Medical Center, Seoul National University H
- Company
- Tabreca can now be prescribed at general hospitals in Korea
- by Eo, Yun-Ho Feb 23, 2023 05:46am
- The first MET-targeted anticancer drug, ‘Tabrecta’ can now be prescribed at hospitals in Korea. According to industry sources, Novartis Kore’s Tabrecta (capmatinib) passed the drug committees of the Big-5s general hospitals including Samsung Medical Center (SMC), Seoul National University Hospital (SNUH), Sinchon Severance Hospital, a
- Company
- Neulasta's sales surpassed those of Neulapeg
- by Kim, Jin-Gu Feb 23, 2023 05:46am
- Neulasta and Neulapeg are competing for the lead in the neutropenia treatment market. In the fourth quarter of 2021, Neulapeg took the lead by surpassing the original product, Neulasta, for the first time after its release, but from the first quarter of last year, Neulasta took the lead again, widening the gap with Neulapeg. In the pharmaceut
- Company
- Celltrion Eyelea expands U.S. patent
- by Feb 22, 2023 05:55am
- Celltrion is conducting an additional trial for invalidation of the U.S. patent for the eye disease treatment "Eylea." According to industries on the 17th, Celltrion recently filed an IPR (Inter Parts Review) with the U.S. Patent and Trademark Office, claiming that Eylea's composition patent developed by Regeneron is invalid. Eylea is a bi
- Company
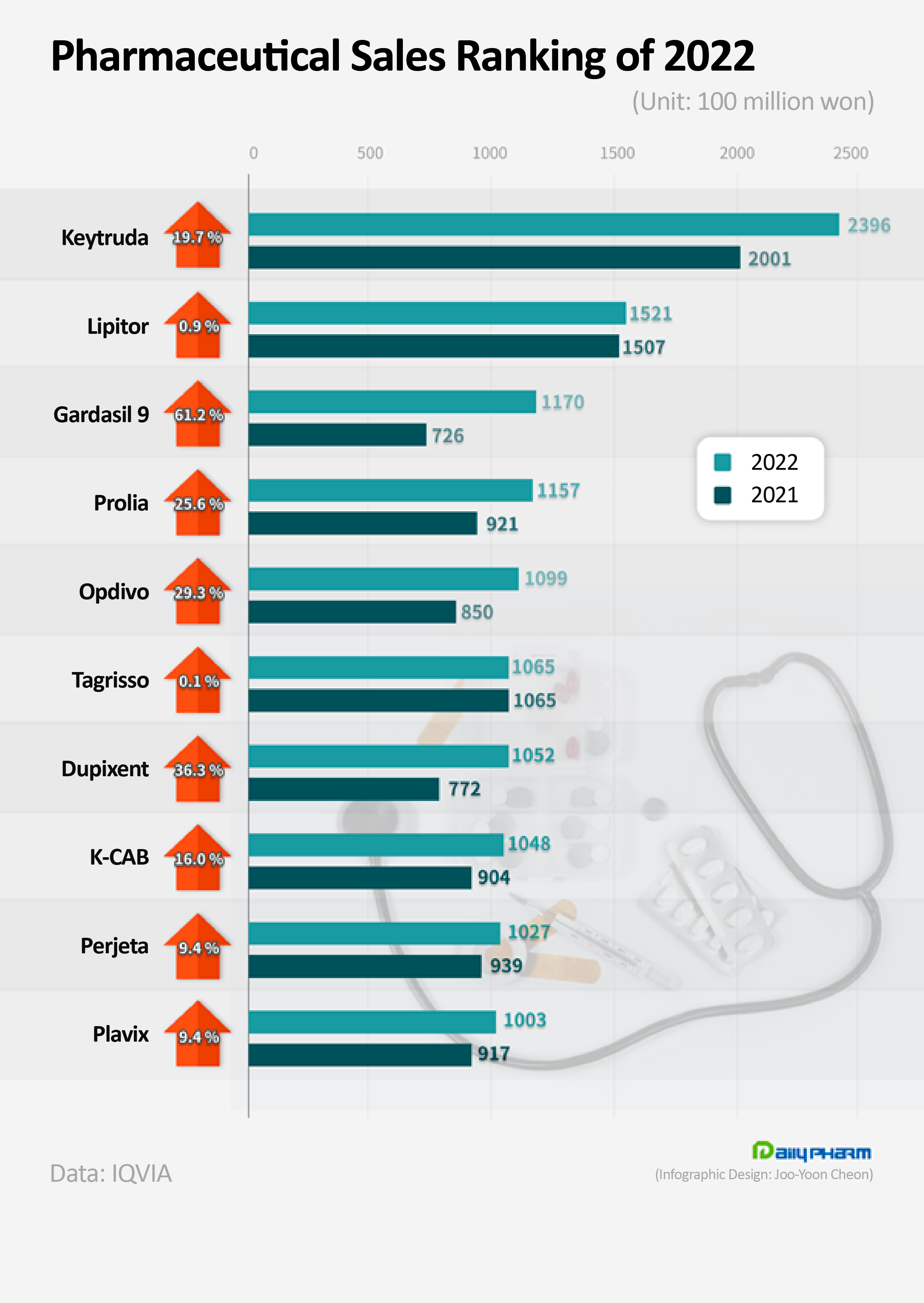
- Keytruda leads the market for 3 consecutive years
- by Chon, Seung-Hyun Feb 22, 2023 05:54am
- The immuno-oncology drug Keytruda has topped the rank in pharmaceutical sales for 3 consecutive years. Also, new drugs from multinational pharmaceutical companies, such as Gardasil 9, Prolia, Opdivo, and Dupixent showed strong growth and joined the KRW 100 billion club after exceeding KRW 100 billion in sales last year. On the 22nd, accordin
- Company
- GC Biopharma receives WHO Pre-Qualification for Barycela
- by Kim, Jin-Gu Feb 21, 2023 05:52am
- GC Biopharma announced that the World Health Organization (WHO) has granted prequalification (PQ) for its varicella vaccine, Barycela. Vaccines that receive the WHO prequalification decision after a review of their safety and efficacy become eligible for procurement by the United Nations agencies to be used in national immunization progra
- Company
- 3rd PARPi Talzenna may be prescribed at hospitals in Korea
- by Eo, Yun-Ho Feb 21, 2023 05:52am
- Korea’s third PARP inhibitor, ‘Talzenna’ can now be prescribed at general hospitals in Korea. According to industry sources, Pfizer Korea’s breast cancer susceptibility gene (BRCA)-mutated (gBRCAm) treatment ‘Talzenna (talazoparib)' passed the drug committee reviews of various medical institutions in Korea, including the National Can
- Company
- Organon ‘Samsung’s Humira biosimilar to make KRW 100 billi
- by Jung, Sae-Im Feb 20, 2023 05:53am
- Samsung Bioepeis’ U.S partner Organon projected that the sales of the company’s Humira biosimilar will reach a maximum of KRW 123.5 billion in the first year of its release. On the 16th (local time), Organon projected so while presenting its full-year 2022 financial results, announcing that the company “will be launching the Humira bio
- Company
- Novartis’s Jakavi makes a step towards reimb for GvHD
- by Eo, Yun-Ho Feb 20, 2023 05:52am
- ‘Jakavi’ is now one step closer to extending reimbursement to Graft versus Host Disease (GvHD) in Korea. According to industry sources, Novartis Kroea’s Jakavi (ruxolitinib) recently passed deliberation by the Drug Reimbursement Evaluation Standard Subcommittee. The next step is for the drug to be reviewed by the Drug Reimbursement Eva